A New "Non-Traditional" Antibacterial Drug Fluorothiazinone-Clinical Research in Patients with Complicated Urinary Tract Infections
- PMID: 38927143
- PMCID: PMC11200362
- DOI: 10.3390/antibiotics13060476
A New "Non-Traditional" Antibacterial Drug Fluorothiazinone-Clinical Research in Patients with Complicated Urinary Tract Infections
Abstract
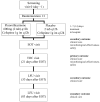
In order to combat resistance, it is necessary to develop antimicrobial agents that act differently from conventional antibiotics. Fluorothiazinone, 300 mg tablet (The Gamaleya National Research Center), is an original antibacterial drug based on a new small molecule T3SS and flagellum inhibitor. A total of 357 patients with complicated urinary tract infections (UTIs) were divided into two groups and given Fluorothiazinone 1200 mg/day or a placebo for 7 days to evaluate the efficacy and safety of the drug. Additionally, all patients were given Cefepime 2000 mg/day. Fluorothiazinone with Cefepime showed superiority over placebo/Cefepime based on the assessment of the proportion of patients with an overall outcome in the form of a cure after 21 days post-therapy (primary outcome), overall outcome in cure rates, clinical cure rates, and microbiological efficacy at the end of therapy and after 21 days post-therapy (secondary outcomes). In patients who received Fluorothiazinone, the rate of infection recurrences 53 and 83 days after the end of the therapy was lower by 18.9%, compared with patients who received placebo. Fluorothiazinone demonstrated a favorable safety profile with no serious unexpected adverse events reported. The results showed superiority of the therapy with Fluorothiazinone in combination with Cefepime compared with placebo/Cefepime in patients with cUTIs.
Keywords: Fluorothiazinone; antibiotic-resistant bacteria; clinical trial; complicated urinary tract infection; non-traditional antibacterial agents; small molecule T3SS and flagella inhibitor.
Conflict of interest statement
Author Konstantin A. Zakharov was employed by the company Accellena. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. 2021. [(accessed on 9 April 2024)]. Available online: https://www.who.int/publications/i/item/9789240047655.
-
- Zigangirova N., Lubenec N., Zaitsev A., Pushkar D. Antibacterial agents reducing the risk of resistance development. CMAC. 2021;23:184–194. doi: 10.36488/cmac.2021.2.184-194. - DOI
-
- Totsika M. Benefits and challenges of antivirulence antimicrobials at the dawn of the post-antibiotic era. Drug Deliv. Lett. 2016;6:30–37. doi: 10.2174/2210303106666160506120057. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

