The Effectiveness of Atezolizumab in Metastatic Large Cell Neuroendocrine Carcinoma of the Lungs: Insights from the LANCE Pilot Study
- PMID: 38927367
- PMCID: PMC11200835
- DOI: 10.3390/biomedicines12061161
The Effectiveness of Atezolizumab in Metastatic Large Cell Neuroendocrine Carcinoma of the Lungs: Insights from the LANCE Pilot Study
Abstract
Background: Large cell neuroendocrine carcinoma (LCNEC) presents significant treatment challenges due to its rarity and limited therapeutic options. The LANCE study was designed to explore the survival benefits of incorporating atezolizumab in chemotherapy for metastatic LCNEC.
Methods: In this non-randomized study, patients with metastatic LCNEC were prospectively enrolled and assigned to receive either standard chemotherapy plus atezolizumab followed by maintenance with atezolizumab or standard chemotherapy alone. The primary outcomes measured were 12- and 24-month survival rates, progression-free survival (PFS), and overall survival (OS) between the two groups.
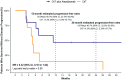
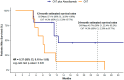
Results: Of the 22 patients screened, 17 met the inclusion criteria and received either atezolizumab plus platinum-based chemotherapy (n = 10) or chemotherapy alone (n = 7). After a median follow-up of 23.3 months, the 12-month survival rate was 57.1% (95% CI: 32.6-100%) and 14.3% (95% CI: 2.33-87.7%) for the atezolizumab and the chemotherapy-only groups, respectively. The survival benefit for the atezolizumab group was sustained at 24 months (45.7% vs. 14.3%). Overall survival was significantly higher for the atezolizumab group, and PFS was non-significantly associated with the addition of atezolizumab (log-rank p = 0.04 and 0.05, respectively).
Conclusions: This pilot study suggests that the addition of atezolizumab to standard platinum-based chemotherapy may provide a substantial survival benefit compared with chemotherapy alone in the first-line treatment of metastatic LCNEC.
Keywords: LCNEC; atezolizumab; immunotherapy; large cell neuroendocrine carcinoma.
Conflict of interest statement
G.E. reports receiving support for attending meetings from ΙPSEN, REGENERON, and institutional fees from Merck and IPSEN. K.S. reports receiving honoraria from Bristol and Amgen, and consulting fees from AstraZeneca and MSD. I.G. reports receiving honoraria from BMS, ROCHE and MSD. The other authors have no conflicts of interest to declare.
Figures



References
-
- Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B., Chirieac L.R., Dacic S., Duhig E., Flieder D.B., et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/jto.0000000000000630. - DOI - PubMed
-
- Paz-Ares L., Dvorkin M., Chen Y., Reinmuth N., Hotta K., Trukhin D., Statsenko G., Hochmair M.J., Özgüroğlu M., Ji J.H., et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. - DOI - PubMed
-
- Yao J.C., Fazio N., Singh S., Buzzoni R., Carnaghi C., Wolin E., Tomasek J., Raderer M., Lahner H., Voi M., et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–977. doi: 10.1016/S0140-6736(15)00817-X. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

