Behavioral Effects of the Mixture and the Single Compounds Carbendazim, Fipronil, and Sulfentrazone on Zebrafish (Danio rerio) Larvae
- PMID: 38927383
- PMCID: PMC11200900
- DOI: 10.3390/biomedicines12061176
Behavioral Effects of the Mixture and the Single Compounds Carbendazim, Fipronil, and Sulfentrazone on Zebrafish (Danio rerio) Larvae
Abstract
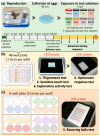
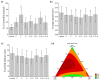
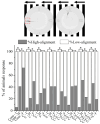
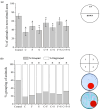
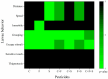
Pesticides are often detected in freshwater, but their impact on the aquatic environment is commonly studied based on single compounds, underestimating the potential additive effects of these mixtures. Even at low concentrations, pesticides can negatively affect organisms, altering important behaviors that can have repercussions at the population level. This study used a multi-behavioral approach to evaluate the effects of zebrafish larvae exposure to carbendazim (C), fipronil (F), and sulfentrazone (S), individually and mixed. Five behavioral tests, thigmotaxis, touch sensitivity, optomotor response, bouncing ball test, and larval exploratory behavior, were performed to assess potential effects on anxiety, fear, and spatial and social interaction. Significant changes were observed in the performance of larvae exposed to all compounds and their mixtures. Among the single pesticides, exposure to S produced the most behavioral alterations, followed by F and C, respectively. A synergistic effect between the compounds was observed in the C + F group, which showed more behavioral effects than the groups exposed to pesticides individually. The use of behavioral tests to evaluate pesticide mixtures is important to standardize methods and associate behavioral changes with ecologically relevant events, thus creating a more realistic scenario for investigating the potential environmental impacts of these compounds.
Keywords: behavior; pesticides; synergism; systemic effect.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Peterson E.K., Buchwalter D.B., Kerby J.L., LeFauve M.K., Varian-Ramos C.W., Swaddle J.P. Integrative behavioral ecotoxicology: Bringing together fields to establish new insight to behavioral ecology, toxicology, and conservation. Curr. Zool. 2017;63:185–194. doi: 10.1093/cz/zox010. - DOI - PMC - PubMed
-
- Severo E.S., Marins A.T., Cerezer C., Costa D., Nunes M., Prestes O.D., Zanella R., Loro V.L. Ecological risk of pesticide contamination in a Brazilian river located near a rural area: A study of biomarkers using zebrafish embryos. Ecotoxicol. Environ. Saf. 2020;190:110071. doi: 10.1016/j.ecoenv.2019.110071. - DOI - PubMed
-
- Li M., Ma X., Saleem M., Wang X., Sun L., Yang Y., Zhang Q. Biochemical response, histopathological change and DNA damage in earthworm (Eisenia fetida) exposed to sulfentrazone herbicide. Ecol. Indic. 2020;115:106465. doi: 10.1016/j.ecolind.2020.106465. - DOI
-
- Kronbauer E.A., Biondo E., Zanetti C., Kolchinski E.M. Agrotóxicos em água do rio e água tratada no Município de Encantado/RS. Ambiente Gestão Desenvolv. 2021;14:6–17. doi: 10.24979/ambiente.v14i2.967. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

