Neurological Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors
- PMID: 38927526
- PMCID: PMC11202292
- DOI: 10.3390/biomedicines12061319
Neurological Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors
Abstract
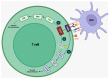
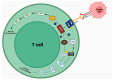
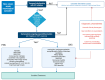
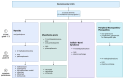
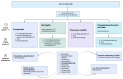
The use of immune checkpoint inhibitors (ICIs) for the treatment of various advanced and aggressive types of malignancy has significantly increased both survival and long-term remission rates. ICIs block crucial inhibitory pathways of the immune system, in order to trigger an aggravated immune response against the tumor. However, this enhanced immune activation leads to the development of numerous immune-related adverse events (irAEs), which may affect any system. Although severe neurological irAEs are relatively rare, they carry a high disability burden, and they can be potentially life-threatening. Therefore, clinicians must be alert and act promptly when individuals receiving ICIs present with new-onset neurological symptoms. In this narrative review, we have collected all the currently available data regarding the epidemiology, pathogenesis, clinical manifestations, diagnosis, and treatment of post-ICI neurological irAEs. This review aims to raise physicians' awareness, enrich their knowledge regarding disease pathogenesis, and guide them through the diagnosis and management of post-ICI neurological irAEs.
Keywords: antineuronal antibodies; central nervous system; immune checkpoint inhibitors; neurological immune-related adverse events; neurotoxicity; paraneoplastic neurological syndromes; peripheral nervous system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Wei S.C., Duffy C.R., Allison J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

