Modeling of Magnetic Scaffolds as Drug Delivery Platforms for Tissue Engineering and Cancer Therapy
- PMID: 38927809
- PMCID: PMC11200873
- DOI: 10.3390/bioengineering11060573
Modeling of Magnetic Scaffolds as Drug Delivery Platforms for Tissue Engineering and Cancer Therapy
Abstract
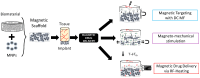
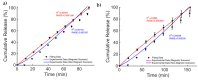
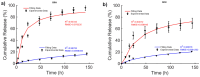
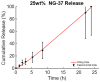
Magnetic scaffolds (MagSs) are magneto-responsive devices obtained by the combination of traditional biomaterials (e.g., polymers, bioceramics, and bioglasses) and magnetic nanoparticles. This work analyzes the literature about MagSs used as drug delivery systems for tissue repair and cancer treatment. These devices can be used as innovative drugs and/or biomolecules delivery systems. Through the application of a static or dynamic stimulus, MagSs can trigger drug release in a controlled and remote way. However, most of MagSs used as drug delivery systems are not optimized and properly modeled, causing a local inhomogeneous distribution of the drug's concentration and burst release. Few physical-mathematical models have been presented to study and analyze different MagSs, with the lack of a systematic vision. In this work, we propose a modeling framework. We modeled the experimental data of drug release from different MagSs, under various magnetic field types, taken from the literature. The data were fitted to a modified Gompertz equation and to the Korsmeyer-Peppas model (KPM). The correlation coefficient (R2) and the root mean square error (RMSE) were the figures of merit used to evaluate the fitting quality. It has been found that the Gompertz model can fit most of the drug delivery cases, with an average RMSE below 0.01 and R2>0.9. This quantitative interpretation of existing experimental data can foster the design and use of MagSs for drug delivery applications.
Keywords: cancer therapy; drug delivery; electromagnetic fields; magnetic nanoparticles; magnetic scaffolds; tissue engineering.
Conflict of interest statement
The authors do not have any conflicts of interest to declare.
Figures


References
-
- de Sousa Victor R., Marcelo da Cunha Santos A., Viana de Sousa B., de Araújo Neves G., Navarro de Lima Santana L., Rodrigues Menezes R. A review on Chitosan’s uses as biomaterial: Tissue engineering, drug delivery systems and cancer treatment. Materials. 2020;13:4995. doi: 10.3390/ma13214995. - DOI - PMC - PubMed
-
- Nour S., Baheiraei N., Imani R., Rabiee N., Khodaei M., Alizadeh A., Moazzeni S.M. Bioactive materials: A comprehensive review on interactions with biological microenvironment based on the immune response. J. Bionic Eng. 2019;16:563–581. doi: 10.1007/s42235-019-0046-z. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

