Immunohistochemical Profiling of SSTR2 and HIF-2α with the Tumor Microenvironment in Pheochromocytoma and Paraganglioma
- PMID: 38927897
- PMCID: PMC11201597
- DOI: 10.3390/cancers16122191
Immunohistochemical Profiling of SSTR2 and HIF-2α with the Tumor Microenvironment in Pheochromocytoma and Paraganglioma
Abstract
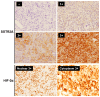
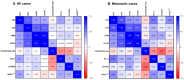
Metastatic pheochromocytomas and paragangliomas (PPGLs) are rare endocrine malignancies with limited effective treatment options. The association between the tumor microenvironment (TME) with somatostatin receptor 2 (SSTR2) and hypoxia-induced factor-2α (HIF-2α) in PPGLs, critical for optimizing combination therapeutic strategies with immunotherapy, remains largely unexplored. To evaluate the association of SSTR2 and HIF-2α immunoreactivity with the TME in patients with PPGLs, we analyzed the expression of SSTR2A, HIF-2α, and TME components, including tumor-infiltrating lymphocytes (CD4 and CD8), tumor-associated macrophages (CD68 and CD163), and PD-L1, using immunohistochemistry in patients with PPGLs. The primary outcome was to determine the association of the immune profiles with SSTR2A and HIF-2α expression. Among 45 patients with PPGLs, SSTR2A and HIF2α were positively expressed in 21 (46.7%) and 14 (31.1%) patients, respectively. The median PD-L1 immunohistochemical score (IHS) was 2.0 (interquartile range: 0-30.0). Positive correlations were observed between CD4, CD8, CD68, and CD163 levels. A negative correlation was found between the CD163/CD68 ratio (an indicator of M2 polarization) and SSTR2A expression (r = -0.385, p = 0.006). HIF-2α expression showed a positive correlation with PD-L1 IHS (r = 0.348, p = 0.013). The co-expression of PD-L1 (HIS > 10) and HIF-2α was found in seven patients (15.6%). No associations were observed between SDHB staining results and the CD163/CD68 ratio, PD-L1, or SSTR2A expression. Our data suggest the potential of combination therapy with immunotherapy and peptide receptor radionuclide therapy or HIF-2α inhibitors as a treatment option in selected PPGL populations.
Keywords: HIF2α; immunotherapy; paraganglioma; pheochromocytoma; somatostatin receptor; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Hescot S., Curras-Freixes M., Deutschbein T., van Berkel A., Vezzosi D., Amar L., de la Fouchardière C., Valdes N., Riccardi F., Do Cao C., et al. Prognosis of malignant pheochromocytoma and paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study. J. Clin. Endocrinol. Metab. 2019;104:2367–2374. doi: 10.1210/jc.2018-01968. - DOI - PubMed
-
- Jimenez C., Subbiah V., Stephen B., Ma J., Milton D., Xu M., Zarifa A., Akhmedzhanov F.O., Tsimberidou A., Habra M.A., et al. Phase II clinical trial of pembrolizumab in patients with progressive metastatic pheochromocytomas and paragangliomas. Cancers. 2020;12:2307. doi: 10.3390/cancers12082307. - DOI - PMC - PubMed
-
- Kao T.W., Bai G.H., Wang T.L., Shih I.M., Chuang C.M., Lo C.L., Tsai M.C., Chiu L.Y., Lin C.C., Shen Y.A. Novel cancer treatment paradigm targeting hypoxia-induced factor in conjunction with current therapies to overcome resistance. J. Exp. Clin. Cancer Res. 2023;42:171. doi: 10.1186/s13046-023-02724-y. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

