Overcoming Treatment Resistance in Medulloblastoma: Underlying Mechanisms and Potential Strategies
- PMID: 38927954
- PMCID: PMC11202166
- DOI: 10.3390/cancers16122249
Overcoming Treatment Resistance in Medulloblastoma: Underlying Mechanisms and Potential Strategies
Abstract
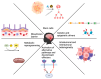
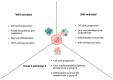
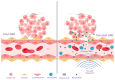
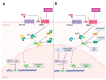
Medulloblastoma is the most frequently encountered malignant brain tumor in the pediatric population. The standard of care currently consists of surgical resection, craniospinal irradiation, and multi-agent chemotherapy. However, despite this combination of multiple aggressive modalities, recurrence of the disease remains a substantial concern, and treatment resistance is a rising issue. The development of this resistance results from the interplay of a myriad of anatomical properties, cellular processes, molecular pathways, and genetic and epigenetic alterations. In fact, several efforts have been directed towards this domain and characterizing the major contributors to this resistance. Herein, this review highlights the different mechanisms that drive relapse and are implicated in the occurrence of treatment resistance and discusses them in the context of the latest molecular-based classification of medulloblastoma. These mechanisms include the impermeability of the blood-brain barrier to drugs, the overactivation of specific molecular pathways, the resistant and multipotent nature of cancer stem cells, intratumoral and intertumoral heterogeneity, and metabolic plasticity. Subsequently, we build on that to explore potential strategies and targeted agents that can abrogate these mechanisms, undermine the development of treatment resistance, and augment medulloblastoma's response to therapeutic modalities.
Keywords: cancer stem cells; combination therapy; medulloblastoma; molecular pathways; treatment resistance; tumoral heterogeneity.
Conflict of interest statement
Betty Tyler has research funding from NIH. Ashvattha Therapeutics Inc. has licensed one of her patents and she is a stockholder for Peabody Pharmaceuticals, which include equity or options.
Figures
References
-
- Ostrom Q.T., Price M., Neff C., Cioffi G., Waite K.A., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro-Oncology. 2023;25((Suppl. S4)):iv1–iv99. doi: 10.1093/neuonc/noad149. - DOI - PMC - PubMed
-
- BAILEY P., Cushing H. Medulloblastoma cerebelli: A common type of midcerebellar glioma of childhood. Arch. Neurol. Psychiatry. 1925;14:192–224. doi: 10.1001/archneurpsyc.1925.02200140055002. - DOI
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

