A Simple and Rapid Microscale Method for Isolating Bacterial Lipopolysaccharides
- PMID: 38928052
- PMCID: PMC11203638
- DOI: 10.3390/ijms25126345
A Simple and Rapid Microscale Method for Isolating Bacterial Lipopolysaccharides
Abstract
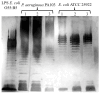
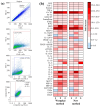
Bacterial endotoxins (lipopolysaccharides (LPSs)) are important mediators of inflammatory processes induced by Gram-negative microorganisms. LPSs are the key inducers of septic shock due to a Gram-negative bacterial infection; thus, the structure and functions of LPSs are of specific interest. Often, highly purified bacterial endotoxins must be isolated from small amounts of biological material. Each of the currently available methods for LPS extraction has certain limitations. Herein, we describe a rapid and simple microscale method for extracting LPSs. The method consists of the following steps: ultrasonic destruction of the bacterial material, LPS extraction via heating, LPS purification with organic solvents, and treatment with proteinase K. LPSs that were extracted by using this method contained less than 2-3% protein and 1% total nucleic acid. We also demonstrated the structural integrity of the O-antigen and lipid A via the sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) methods, respectively. We demonstrated the ability of the extracted LPSs to induce typical secretion of cytokines and chemokines by primary macrophages. Overall, this method may be used to isolate purified LPSs with preserved structures of both the O-antigen and lipid A and unchanged functional activity from small amounts of bacterial biomass.
Keywords: bacterial endotoxin; cell signaling; cytokines; inflammation; lipopolysaccharides; mass spectrometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Coats S.R., Pham T.T.T., Bainbridge B.W., Reife R.A., Darveau R.P. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J. Immunol. 2005;175:4490–4498. doi: 10.4049/jimmunol.175.7.4490. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

