Heat Stress and Microbial Stress Induced Defensive Phenol Accumulation in Medicinal Plant Sparganium stoloniferum
- PMID: 38928085
- PMCID: PMC11203919
- DOI: 10.3390/ijms25126379
Heat Stress and Microbial Stress Induced Defensive Phenol Accumulation in Medicinal Plant Sparganium stoloniferum
Abstract
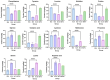
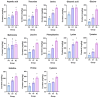
An approach based on the heat stress and microbial stress model of the medicinal plant Sparganium stoloniferum was proposed to elucidate the regulation and mechanism of bioactive phenol accumulation. This method integrates LC-MS/MS analysis, 16S rRNA sequencing, RT-qPCR, and molecular assays to investigate the regulation of phenolic metabolite biosynthesis in S. stoloniferum rhizome (SL) under stress. Previous research has shown that the metabolites and genes involved in phenol biosynthesis correlate to the upregulation of genes involved in plant-pathogen interactions. High-temperature and the presence of Pseudomonas bacteria were observed alongside SL growth. Under conditions of heat stress or Pseudomonas bacteria stress, both the metabolites and genes involved in phenol biosynthesis were upregulated. The regulation of phenol content and phenol biosynthesis gene expression suggests that phenol-based chemical defense of SL is stimulated under stress. Furthermore, the rapid accumulation of phenolic substances relied on the consumption of amino acids. Three defensive proteins, namely Ss4CL, SsC4H, and SsF3'5'H, were identified and verified to elucidate phenol biosynthesis in SL. Overall, this study enhances our understanding of the phenol-based chemical defense of SL, indicating that bioactive phenol substances result from SL's responses to the environment and providing new insights for growing the high-phenol-content medicinal herb SL.
Keywords: Sparganium stoloniferum; amino acid; chemical defense; heat stress; microbial stress; phenol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










Similar articles
-
Integration of 16 S rRNA gene sequencing, metabonomics and metagenome analysis to investigate the mechanism of Sparganium stoloniferum-Curcuma phaeocaulis in treating of endometriosis in rats.J Pharm Biomed Anal. 2024 Apr 15;241:115970. doi: 10.1016/j.jpba.2024.115970. Epub 2024 Jan 17. J Pharm Biomed Anal. 2024. PMID: 38277707
-
Full-length transcriptome analysis of Coptis deltoidea and identification of putative genes involved in benzylisoquinoline alkaloids biosynthesis based on combined sequencing platforms.Plant Mol Biol. 2020 Mar;102(4-5):477-499. doi: 10.1007/s11103-019-00959-y. Epub 2020 Jan 4. Plant Mol Biol. 2020. PMID: 31902069
-
UV-B Radiation Largely Promoted the Transformation of Primary Metabolites to Phenols in Astragalus mongholicus Seedlings.Biomolecules. 2020 Mar 26;10(4):504. doi: 10.3390/biom10040504. Biomolecules. 2020. PMID: 32225015 Free PMC article.
-
Molecular insights into sensing, regulation and improving of heat tolerance in plants.Plant Cell Rep. 2022 Mar;41(3):799-813. doi: 10.1007/s00299-021-02793-3. Epub 2021 Oct 21. Plant Cell Rep. 2022. PMID: 34676458 Review.
-
Physiological and omics-based insights for underpinning the molecular regulation of secondary metabolite production in medicinal plants: UV stress resilience.Plant Physiol Biochem. 2023 Nov;204:108060. doi: 10.1016/j.plaphy.2023.108060. Epub 2023 Oct 27. Plant Physiol Biochem. 2023. PMID: 37897892 Review.
Cited by
-
The rhizobacterial Priestia megaterium strain SH-19 mitigates the hazardous effects of heat stress via an endogenous secondary metabolite elucidation network and molecular regulation signalling.BMC Plant Biol. 2024 Sep 4;24(1):827. doi: 10.1186/s12870-024-05534-2. BMC Plant Biol. 2024. PMID: 39227801 Free PMC article.
References
-
- Parida A.K., Dagaonkar V.S., Phalak M.S., Umalkar G.V., Aurangabadkar L.P. Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotechnol. Rep. 2007;1:37–48. doi: 10.1007/s11816-006-0004-1. - DOI
-
- Divekar P.A., Narayana S., Divekar B.A., Kumar R., Gadratagi B.G., Ray A., Singh A.K., Rani V., Singh V., Singh A.K., et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022;23:2690. doi: 10.3390/ijms23052690. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

