Mechanisms Underlying the Effects of Chloroquine on Red Blood Cells Metabolism
- PMID: 38928131
- PMCID: PMC11203553
- DOI: 10.3390/ijms25126424
Mechanisms Underlying the Effects of Chloroquine on Red Blood Cells Metabolism
Abstract
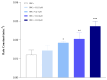
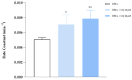
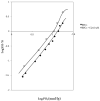
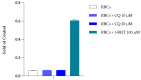
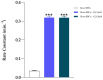
Chloroquine (CQ) is a 4-aminoquinoline derivative largely employed in the management of malaria. CQ treatment exploits the drug's ability to cross the erythrocyte membrane, inhibiting heme polymerase in malarial trophozoites. Accumulation of CQ prevents the conversion of heme to hemozoin, causing its toxic buildup, thus blocking the survival of Plasmodium parasites. Recently, it has been reported that CQ is able to exert antiviral properties, mainly against HIV and SARS-CoV-2. This renewed interest in CQ treatment has led to the development of new studies which aim to explore its side effects and long-term outcome. Our study focuses on the effects of CQ in non-parasitized red blood cells (RBCs), investigating hemoglobin (Hb) functionality, the anion exchanger 1 (AE1) or band 3 protein, caspase 3 and protein tyrosine phosphatase 1B (PTP-1B) activity, intra and extracellular ATP levels, and the oxidative state of RBCs. Interestingly, CQ influences the functionality of both Hb and AE1, the main RBC proteins, affecting the properties of Hb oxygen affinity by shifting the conformational structure of the molecule towards the R state. The influence of CQ on AE1 flux leads to a rate variation of anion exchange, which begins at a concentration of 2.5 μM and reaches its maximum effect at 20 µM. Moreover, a significant decrease in intra and extracellular ATP levels was observed in RBCs pre-treated with 10 µM CQ vs. erythrocytes under normal conditions. This effect is related to the PTP-1B activity which is reduced in RBCs incubated with CQ. Despite these metabolic alterations to RBCs caused by exposure to CQ, no signs of variations in oxidative state or caspase 3 activation were recorded. Our results highlight the antithetical effects of CQ on the functionality and metabolism of RBCs, and encourage the development of new research to better understand the multiple potentiality of the drug.
Keywords: antioxidant systems; band 3 protein; chloroquine; hemoglobin; oxygenation–deoxygenation cycle; red blood cells; sulfate transport.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

