Impact of Serotonin Deficiency on Circadian Dopaminergic Rhythms
- PMID: 38928178
- PMCID: PMC11203511
- DOI: 10.3390/ijms25126475
Impact of Serotonin Deficiency on Circadian Dopaminergic Rhythms
Abstract
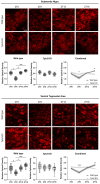
Physiology and behavior are structured temporally to anticipate daily cycles of light and dark, ensuring fitness and survival. Neuromodulatory systems in the brain-including those involving serotonin and dopamine-exhibit daily oscillations in neural activity and help shape circadian rhythms. Disrupted neuromodulation can cause circadian abnormalities that are thought to underlie several neuropsychiatric disorders, including bipolar mania and schizophrenia, for which a mechanistic understanding is still lacking. Here, we show that genetically depleting serotonin in Tph2 knockout mice promotes manic-like behaviors and disrupts daily oscillations of the dopamine biosynthetic enzyme tyrosine hydroxylase (TH) in midbrain dopaminergic nuclei. Specifically, while TH mRNA and protein levels in the Substantia Nigra (SN) and Ventral Tegmental Area (VTA) of wild-type mice doubled between the light and dark phase, TH levels were high throughout the day in Tph2 knockout mice, suggesting a hyperdopaminergic state. Analysis of TH expression in striatal terminal fields also showed blunted rhythms. Additionally, we found low abundance and blunted rhythmicity of the neuropeptide cholecystokinin (Cck) in the VTA of knockout mice, a neuropeptide whose downregulation has been implicated in manic-like states in both rodents and humans. Altogether, our results point to a previously unappreciated serotonergic control of circadian dopamine signaling and propose serotonergic dysfunction as an upstream mechanism underlying dopaminergic deregulation and ultimately maladaptive behaviors.
Keywords: bipolar disorders; cholecystokinin; circadian rhythms; dopamine; hyperdopaminergia; manic-like behavior; serotonin; tryptophan hydroxylase 2; tyrosine hydroxylase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- 953327/EU H2020 MSCA ITN Project "Serotonin and Beyond"
- ECS 00000017/Next Generation EU National Recovery and Resilience Plan, the Ministry of University and Research ("Tuscany Health Ecosystem THE", Spoke 8)
- NA/MIT-Italy seed funds support
- 23771/AFM-Telethon Grant
- Department of Excellence 2023-2027/Italian Ministry of Education, University and Research (MIUR)
LinkOut - more resources
Full Text Sources

