Modulation of Photosystem II Function in Celery via Foliar-Applied Salicylic Acid during Gradual Water Deficit Stress
- PMID: 38928427
- PMCID: PMC11203862
- DOI: 10.3390/ijms25126721
Modulation of Photosystem II Function in Celery via Foliar-Applied Salicylic Acid during Gradual Water Deficit Stress
Abstract
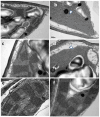
Water deficit is the major stress factor magnified by climate change that causes the most reductions in plant productivity. Knowledge of photosystem II (PSII) response mechanisms underlying crop vulnerability to drought is critical to better understanding the consequences of climate change on crop plants. Salicylic acid (SA) application under drought stress may stimulate PSII function, although the exact mechanism remains essentially unclear. To reveal the PSII response mechanism of celery plants sprayed with water (WA) or SA, we employed chlorophyll fluorescence imaging analysis at 48 h, 96 h, and 192 h after watering. The results showed that up to 96 h after watering, the stroma lamellae of SA-sprayed leaves appeared dilated, and the efficiency of PSII declined, compared to WA-sprayed plants, which displayed a better PSII function. However, 192 h after watering, the stroma lamellae of SA-sprayed leaves was restored, while SA boosted chlorophyll synthesis, and by ameliorating the osmotic potential of celery plants, it resulted in higher relative leaf water content compared to WA-sprayed plants. SA, by acting as an antioxidant under drought stress, suppressed phototoxicity, thereby offering PSII photoprotection, together with enhanced effective quantum yield of PSII photochemistry (ΦPSII) and decreased quantity of singlet oxygen (1O2) generation compared to WA-sprayed plants. The PSII photoprotection mechanism induced by SA under drought stress was triggered by non-photochemical quenching (NPQ), which is a strategy to protect the chloroplast from photo-oxidative damage by dissipating the excess light energy as heat. This photoprotective mechanism, triggered by NPQ under drought stress, was adequate in keeping, especially in high-light conditions, an equal fraction of open PSII reaction centers (qp) as of non-stress conditions. Thus, under water deficit stress, SA activates a regulatory network of stress and light energy partitioning signaling that can mitigate, to an extent, the water deficit stress on PSII functioning.
Keywords: chlorophyll fluorescence imaging; chloroplast ultrastructure; drought; electron transport rate; photochemical quenching; photoprotective heat dissipation; singlet oxygen.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Fregonezi B.F., Pereira A.E.S., Ferreira J.M., Fraceto L.F., Gomes D.G., Oliveira H.C. Seed priming with nanoencapsulated gibberellic acid triggers beneficial morphophysiological and biochemical responses of tomato plants under different water conditions. Agronomy. 2024;14:588. doi: 10.3390/agronomy14030588. - DOI
-
- Wing I.S., De Cian E., Mistry M.N. Global vulnerability of crop yields to climate change. J. Environ. Econ. Manag. 2021;109:102462. doi: 10.1016/j.jeem.2021.102462. - DOI
-
- Placide R., Hirut G.B., Stephan N., Fekadu B. Assessment of drought stress tolerance in root and tuber crops. Afr. J. Plant Sci. 2014;8:214–224. doi: 10.5897/AJPS2014.1169. - DOI
-
- Sperdouli I., Mellidou I., Moustakas M. Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience. Climate. 2021;9:154. doi: 10.3390/cli9110154. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

