Discovery of Di(het)arylmethane and Dibenzoxanthene Derivatives as Potential Anticancer Agents
- PMID: 38928428
- PMCID: PMC11203978
- DOI: 10.3390/ijms25126724
Discovery of Di(het)arylmethane and Dibenzoxanthene Derivatives as Potential Anticancer Agents
Abstract
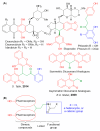
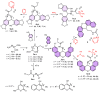
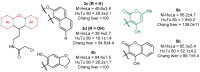
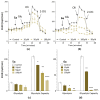
A family of bifunctional dihetarylmethanes and dibenzoxanthenes is assembled via a reaction of acetals containing a 2-chloroacetamide moiety with phenols and related oxygen-containing heterocycles. These compounds demonstrated selective antitumor activity associated with the induction of cell apoptosis and inhibition of the process of glycolysis. In particular, bis(heteroaryl)methane containing two 4-hydroxy-6-methyl-2H-pyran-2-one moieties combine excellent in vitro antitumor efficacy with an IC50 of 1.7 µM in HuTu-80 human duodenal adenocarcinoma models with a high selectivity index of 73. Overall, this work highlights the therapeutic potential of dimeric compounds assembled from functionalized acetals and builds a starting point for the development of a new family of anticancer agents.
Keywords: anticancer agents; cytotoxic activity; di(het)arylmethane; dibenzoxanthene.
Conflict of interest statement
Author Nurbol Appazov was employed by the company Limited Liability Partnership «DPS-Kyzylorda». The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Hassanpour S.H., Dehghani M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017;4:127–129. doi: 10.1016/j.jcrpr.2017.07.001. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

