Inflamma-miRs Profile in Myelodysplastic Syndrome Patients
- PMID: 38928489
- PMCID: PMC11204089
- DOI: 10.3390/ijms25126784
Inflamma-miRs Profile in Myelodysplastic Syndrome Patients
Abstract
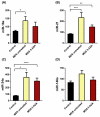
Etiological factors involved in myelodysplastic syndrome (MDS) include immunologic, oxidative stress and inflammatory factors, among others, and these are targets for microRNAs (miRNs). Here, we evaluated whether some miRNs may affect tumor development comparing untreated and 5-azacitidine (5-AZA) MDS-treated patients. Peripheral blood samples were collected from 20 controls and 24 MDS patients, and selected miRNs related to redox balance and inflammation (inflamma-miRs), including miR-18a, miR-21, miR-34a and miR-146a, were isolated and measured by quantitative real-time polymerase chain reaction (qRTPCR). A differential expression profile of miRNs was detected in untreated MDS patients and the 5-AZA group. Inflammation increases miRNs and, specifically, miR-18a, miR-21 and miR-34a were significantly overexpressed in untreated MDS, compared to controls. However, we did not observe any miRN profile alteration during the progression of the disease. On the other hand, 5-AZA treatment tends to restore miRN expression levels. Relating to prognostic risk factors, high-risk MDS groups (high Revised International Prognostic Scoring System (IPSS-R), high cytogenetic risk, high molecular risk (HMR) mutations) tended to be related with higher expression levels of miR-18a and miR-34a. Higher miRN expression is correlated with lower glutathione peroxidase activity, while they are related with a higher profile of pro-inflammatory cytokines (IL-2, IL-6, IL-8, TNF-α). Although our study was limited by the low number of MDS patients included, we identified miRN deregulation involved in MDS development that could regulate redox sensors and inflammatory responses. Finally, 5-AZA treatment is related with lower miRN expression levels in MDS patients.
Keywords: 5-azacitidine (5-AZA); inflammatory cytokines; microRNAs (miRNs); myelodysplastic syndrome (MDS); oxidative stress.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Montes P., Guerra-Librero A., García P., Cornejo-Calvo M.E., López M.D., Haro T.D., Martínez-Ruiz L., Escames G., Acuña-Castroviejo D. Effect of 5-Azacitidine Treatment on Redox Status and Inflammatory Condition in MDS Patients. Antioxidants. 2022;11:139. doi: 10.3390/antiox11010139. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

