The Extract of Gloiopeltis tenax Enhances Myogenesis and Alleviates Dexamethasone-Induced Muscle Atrophy
- PMID: 38928510
- PMCID: PMC11203874
- DOI: 10.3390/ijms25126806
The Extract of Gloiopeltis tenax Enhances Myogenesis and Alleviates Dexamethasone-Induced Muscle Atrophy
Abstract
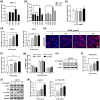
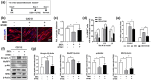
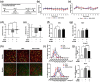
The decline in the function and mass of skeletal muscle during aging or other pathological conditions increases the incidence of aging-related secondary diseases, ultimately contributing to a decreased lifespan and quality of life. Much effort has been made to surmise the molecular mechanisms underlying muscle atrophy and develop tools for improving muscle function. Enhancing mitochondrial function is considered critical for increasing muscle function and health. This study is aimed at evaluating the effect of an aqueous extract of Gloiopeltis tenax (GTAE) on myogenesis and muscle atrophy caused by dexamethasone (DEX). The GTAE promoted myogenic differentiation, accompanied by an increase in peroxisome proliferator-activated receptor γ coactivator α (PGC-1α) expression and mitochondrial content in myoblast cell culture. In addition, the GTAE alleviated the DEX-mediated myotube atrophy that is attributable to the Akt-mediated inhibition of the Atrogin/MuRF1 pathway. Furthermore, an in vivo study using a DEX-induced muscle atrophy mouse model demonstrated the efficacy of GTAE in protecting muscles from atrophy and enhancing mitochondrial biogenesis and function, even under conditions of atrophy. Taken together, this study suggests that the GTAE shows propitious potential as a nutraceutical for enhancing muscle function and preventing muscle wasting.
Keywords: Gloiopeltis tenax; PGC-1α; mitochondria; muscle atrophy; red algae.
Conflict of interest statement
H.E.P., H.-I.J. and J.L. were employed by Redone Technologies Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Vigeo Promotes Myotube Differentiation and Protects Dexamethasone-Induced Skeletal Muscle Atrophy via Regulating the Protein Degradation, AKT/mTOR, and AMPK/Sirt-1/PGC1α Signaling Pathway In Vitro and In Vivo.Nutrients. 2024 Aug 13;16(16):2687. doi: 10.3390/nu16162687. Nutrients. 2024. PMID: 39203823 Free PMC article.
-
Dual Action of Pueraria montana var. lobata Extract on Myogenesis and Muscle Atrophy.Nutrients. 2025 Mar 30;17(7):1217. doi: 10.3390/nu17071217. Nutrients. 2025. PMID: 40218975 Free PMC article.
-
Dihydromyricetin Attenuates Dexamethasone-Induced Muscle Atrophy by Improving Mitochondrial Function via the PGC-1α Pathway.Cell Physiol Biochem. 2018;49(2):758-779. doi: 10.1159/000493040. Epub 2018 Aug 30. Cell Physiol Biochem. 2018. PMID: 30165349
-
The Alleviative Effect of Sodium Butyrate on Dexamethasone-Induced Skeletal Muscle Atrophy.Cell Biol Int. 2025 May;49(5):508-521. doi: 10.1002/cbin.70003. Epub 2025 Feb 12. Cell Biol Int. 2025. PMID: 39936899
-
Ameliorative Effects of Fermented Red Ginseng Extract on Muscle Atrophy in Dexamethasone-Induced C2C12 Cell And Hind Limb-Immobilized C57BL/6J Mice.J Med Food. 2024 Oct;27(10):951-960. doi: 10.1089/jmf.2024.k.0168. Epub 2024 Aug 21. J Med Food. 2024. PMID: 39167545
Cited by
-
Marine-Derived Bioactive Compounds: A Promising Strategy for Ameliorating Skeletal Muscle Dysfunction in COPD.Mar Drugs. 2025 Apr 4;23(4):158. doi: 10.3390/md23040158. Mar Drugs. 2025. PMID: 40278279 Free PMC article. Review.
References
-
- Stellavato A., Abate L., Vassallo V., Donniacuo M., Rinaldi B., Schiraldi C. An in vitro study to assess the effect of hyaluronan-based gels on muscle-derived cells: Highlighting a new perspective in regenerative medicine. PLoS ONE. 2020;15:e0236164. doi: 10.1371/journal.pone.0236164. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

