Protein Oxidative Modifications in Neurodegenerative Diseases: From Advances in Detection and Modelling to Their Use as Disease Biomarkers
- PMID: 38929122
- PMCID: PMC11200609
- DOI: 10.3390/antiox13060681
Protein Oxidative Modifications in Neurodegenerative Diseases: From Advances in Detection and Modelling to Their Use as Disease Biomarkers
Abstract
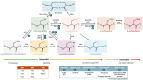
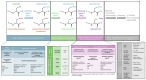
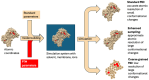
Oxidation-reduction post-translational modifications (redox-PTMs) are chemical alterations to amino acids of proteins. Redox-PTMs participate in the regulation of protein conformation, localization and function, acting as signalling effectors that impact many essential biochemical processes in the cells. Crucially, the dysregulation of redox-PTMs of proteins has been implicated in the pathophysiology of numerous human diseases, including neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease. This review aims to highlight the current gaps in knowledge in the field of redox-PTMs biology and to explore new methodological advances in proteomics and computational modelling that will pave the way for a better understanding of the role and therapeutic potential of redox-PTMs of proteins in neurodegenerative diseases. Here, we summarize the main types of redox-PTMs of proteins while providing examples of their occurrence in neurodegenerative diseases and an overview of the state-of-the-art methods used for their detection. We explore the potential of novel computational modelling approaches as essential tools to obtain insights into the precise role of redox-PTMs in regulating protein structure and function. We also discuss the complex crosstalk between various PTMs that occur in living cells. Finally, we argue that redox-PTMs of proteins could be used in the future as diagnosis and prognosis biomarkers for neurodegenerative diseases.
Keywords: Alzheimer’s disease; Parkinson’s disease; biomarkers; computational modelling; molecular dynamics; neurodegenerative diseases; redox post-translational modifications; redox proteomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
Grants and funding
- 2021.04378.CEECIND/Fundação para a Ciência e Tecnologia
- EXPL/BTM-TEC/1407/2021/Fundação para a Ciência e Tecnologia
- 218466/Z/19/Z/Wellcome 4-year PhD Programme in Science Drug Discovery and Team Science
- DL57/2016/CP1376/CT0009/Fundação para a Ciência e Tecnologia
- EXPL/BIA-BQM/0793/2021/Fundação para a Ciência e Tecnologia
LinkOut - more resources
Full Text Sources
Miscellaneous

