Ergothioneine-Mediated Neuroprotection of Human iPSC-Derived Dopaminergic Neurons
- PMID: 38929132
- PMCID: PMC11200999
- DOI: 10.3390/antiox13060693
Ergothioneine-Mediated Neuroprotection of Human iPSC-Derived Dopaminergic Neurons
Abstract
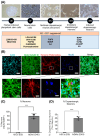
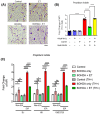
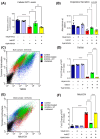
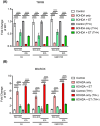
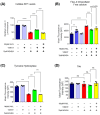
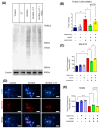
Cell death involving oxidative stress and mitochondrial dysfunction is a major cause of dopaminergic neuronal loss in the substantia nigra (SN) of Parkinson's disease patients. Ergothioneine (ET), a natural dietary compound, has been shown to have cytoprotective functions, but neuroprotective actions against PD have not been well established. 6-Hydroxydopamine (6-OHDA) is a widely used neurotoxin to simulate the degeneration of dopaminergic (DA) neurons in Parkinson's disease. In this study, we investigated the protective effect of ET on 6-OHDA treated iPSC-derived dopaminergic neurons (iDAs) and further confirmed the protective effects in 6-OHDA-treated human neuroblastoma SH-SY5Y cells. In 6-OHDA-treated cells, decreased mitochondrial membrane potential (ΔΨm), increased mitochondrial reactive oxygen species (mROS), reduced cellular ATP levels, and increased total protein carbonylation levels were observed. 6-OHDA treatment also significantly decreased tyrosine hydroxylase levels. These effects were significantly decreased when ET was present. Verapamil hydrochloride (VHCL), a non-specific inhibitor of the ET transporter OCTN1 abrogated ET's cytoprotective effects, indicative of an intracellular action. These results suggest that ET could be a potential therapeutic for Parkinson's disease.
Keywords: 6-OHDA; Parkinson’s disease; ergothioneine; mitochondrial dysfunction; neurodegeneration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

