Liver Cell Mitophagy in Metabolic Dysfunction-Associated Steatotic Liver Disease and Liver Fibrosis
- PMID: 38929168
- PMCID: PMC11200567
- DOI: 10.3390/antiox13060729
Liver Cell Mitophagy in Metabolic Dysfunction-Associated Steatotic Liver Disease and Liver Fibrosis
Abstract
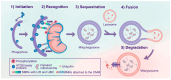
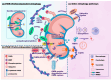
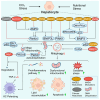
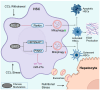
Metabolic dysfunction-associated steatotic liver disease (MASLD) affects approximately one-third of the global population. MASLD and its advanced-stage liver fibrosis and cirrhosis are the leading causes of liver failure and liver-related death worldwide. Mitochondria are crucial organelles in liver cells for energy generation and the oxidative metabolism of fatty acids and carbohydrates. Recently, mitochondrial dysfunction in liver cells has been shown to play a vital role in the pathogenesis of MASLD and liver fibrosis. Mitophagy, a selective form of autophagy, removes and recycles impaired mitochondria. Although significant advances have been made in understanding mitophagy in liver diseases, adequate summaries concerning the contribution of liver cell mitophagy to MASLD and liver fibrosis are lacking. This review will clarify the mechanism of liver cell mitophagy in the development of MASLD and liver fibrosis, including in hepatocytes, macrophages, hepatic stellate cells, and liver sinusoidal endothelial cells. In addition, therapeutic strategies or compounds related to hepatic mitophagy are also summarized. In conclusion, mitophagy-related therapeutic strategies or compounds might be translational for the clinical treatment of MASLD and liver fibrosis.
Keywords: Kupffer cells (KCs); hepatic stellate cells (HSCs); hepatocytes; liver fibrosis; liver sinusoidal endothelial cells (LSECs); macrophages; metabolic dysfunction-associated steatohepatitis (MASH); metabolic dysfunction-associated steatotic liver disease (MASLD); mitochondria; mitophagy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Liver sinusoidal endothelial cells: Friend or foe in metabolic dysfunction- associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis.Dig Liver Dis. 2025 May;57(5):493-503. doi: 10.1016/j.dld.2025.01.189. Epub 2025 Feb 3. Dig Liver Dis. 2025. PMID: 39904692 Review.
-
MASLD development: From molecular pathogenesis toward therapeutic strategies.Chin Med J (Engl). 2025 Aug 5;138(15):1807-1824. doi: 10.1097/CM9.0000000000003629. Epub 2025 Jul 10. Chin Med J (Engl). 2025. PMID: 40640088 Free PMC article. Review.
-
Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases.Front Cell Dev Biol. 2024 Jul 16;12:1433857. doi: 10.3389/fcell.2024.1433857. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39086662 Free PMC article. Review.
-
STING Activation in Various Cell Types in Metabolic Dysfunction-Associated Steatotic Liver Disease.Liver Int. 2025 Apr;45(4):e70063. doi: 10.1111/liv.70063. Liver Int. 2025. PMID: 40116753 Review.
-
Aging-Associated Liver Sinusoidal Endothelial Cells Dysfunction Aggravates the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease.Aging Cell. 2025 May;24(5):e14502. doi: 10.1111/acel.14502. Epub 2025 Feb 6. Aging Cell. 2025. PMID: 39912563 Free PMC article.
Cited by
-
Green Tea Polyphenol (-)-Epicatechin Pretreatment Mitigates Hepatic Steatosis in an In Vitro MASLD Model.Curr Issues Mol Biol. 2024 Aug 16;46(8):8981-8994. doi: 10.3390/cimb46080531. Curr Issues Mol Biol. 2024. PMID: 39194748 Free PMC article.
-
Apolipoprotein L2's Role in Liver Fibrosis.Gastro Hep Adv. 2025 Mar 27;4(7):100665. doi: 10.1016/j.gastha.2025.100665. eCollection 2025. Gastro Hep Adv. 2025. PMID: 40503184 Free PMC article. No abstract available.
-
Single-cell transcriptome analyses reveal the mechanism of mitochondrial activity in erectile dysfunction.Sex Med. 2025 Jul 20;13(3):qfaf049. doi: 10.1093/sexmed/qfaf049. eCollection 2025 Jun. Sex Med. 2025. PMID: 40689149 Free PMC article.
-
Mechanism of Lipi Jiangzhuo Decoction in Improving Metabolic Dysfunction-Associated Steatohepatitis Through the PERK/PINK1/GPx4 Pathway.J Inflamm Res. 2025 Aug 13;18:10969-10994. doi: 10.2147/JIR.S532630. eCollection 2025. J Inflamm Res. 2025. PMID: 40827263 Free PMC article.
-
Mitochondrial Dysfunction as a Pathogenesis and Therapeutic Strategy for Metabolic-Dysfunction-Associated Steatotic Liver Disease.Int J Mol Sci. 2025 Apr 30;26(9):4256. doi: 10.3390/ijms26094256. Int J Mol Sci. 2025. PMID: 40362504 Free PMC article. Review.
References
-
- Chan K.E., Ong E.Y.H., Chung C.H., Ong C.E.Y., Koh B., Tan D.J.H., Lim W.H., Yong J.N., Xiao J., Wong Z.Y., et al. Longitudinal Outcomes Associated With Metabolic Dysfunction-Associated Steatotic Liver Disease: A Meta-analysis of 129 Studies. Clin. Gastroenterol. Hepatol. 2024;22:488–498.e414. doi: 10.1016/j.cgh.2023.09.018. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

