Congenital Lung Malformations: A Pictorial Review of Imaging Findings and a Practical Guide for Diagnosis
- PMID: 38929218
- PMCID: PMC11201397
- DOI: 10.3390/children11060638
Congenital Lung Malformations: A Pictorial Review of Imaging Findings and a Practical Guide for Diagnosis
Abstract
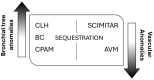
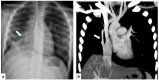
The term congenital lung malformation (CLM) is used to describe a wide range of pathological conditions with different imaging and clinical manifestations. These anomalies stem from abnormal embryological lung development, potentially occurring across various stages of prenatal life. Their natural history can be variable, presenting in a wide range of severity levels and encompassing asymptomatic individuals who remain so until adulthood, as well as those who experience respiratory distress in the neonatal period. Through the PubMed database, we performed an extensive review of the literature in the fields of congenital lung abnormalities, including their diagnostic approach and findings. From our RIS-PACS database, we have selected cases with a final diagnosis of congenital lung malformation. Different diagnostic approaches have been selected, including clinical cases studied using plain radiograph, CT scan, prenatal ultrasound, and MR images. The most encountered anomalies can be classified into three categories: bronchopulmonary anomalies (congenital pulmonary airway malformations (CPAMs), congenital lobar hyperinflation, bronchial atresia, and bronchogenic cysts), vascular anomalies (arteriovenous malformation), and combined lung and vascular anomalies (scimitar syndrome and bronchopulmonary sequestration). CLM causes significant morbidity and mortality; therefore, the recognition of these abnormalities is necessary for optimal prenatal counseling and early peri- and postnatal management. This pictorial review aims to report relevant imaging findings in order to offer some clues for differential diagnosis both for radiologists and pediatric consultants.
Keywords: MR imaging; computed tomography; congenital lung anomalies; congenital lung malformations; congenital thoracic malformations; imaging evaluation; imaging guidelines; radiography; ultrasound.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures















References
Publication types
LinkOut - more resources
Full Text Sources

