The Complement System as a Therapeutic Target in Retinal Disease
- PMID: 38929562
- PMCID: PMC11205777
- DOI: 10.3390/medicina60060945
The Complement System as a Therapeutic Target in Retinal Disease
Abstract
The complement cascade is a vital system in the human body's defense against pathogens. During the natural aging process, it has been observed that this system is imperative for ensuring the integrity and homeostasis of the retina. While this system is critical for proper host defense and retinal integrity, it has also been found that dysregulation of this system may lead to certain retinal pathologies, including geographic atrophy and diabetic retinopathy. Targeting components of the complement system for retinal diseases has been an area of interest, and in vivo, ex vivo, and clinical trials have been conducted in this area. Following clinical trials, medications targeting the complement system for retinal disease have also become available. In this manuscript, we discuss the pathophysiology of complement dysfunction in the retina and specific pathologies. We then describe the results of cellular, animal, and clinical studies targeting the complement system for retinal diseases. We then provide an overview of complement inhibitors that have been approved by the Food and Drug Administration (FDA) for geographic atrophy. The complement system in retinal diseases continues to serve as an emerging therapeutic target, and further research in this field will provide additional insights into the mechanisms and considerations for treatment of retinal pathologies.
Keywords: age-related macular degeneration; choroid; complement system; retinal therapy.
Conflict of interest statement
JC: Allergan, Salutaris, Biogen, Erasca. J.C. and J.O. served as guest editors in Medicina at time of submission. All author authors declare no conflicts of interest.
Figures



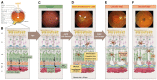
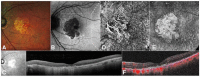
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

