Vitamin K2 Supplementation in Hospitalised COVID-19 Patients: A Randomised Controlled Trial
- PMID: 38930004
- PMCID: PMC11205124
- DOI: 10.3390/jcm13123476
Vitamin K2 Supplementation in Hospitalised COVID-19 Patients: A Randomised Controlled Trial
Abstract
Background: In observational studies, high levels of desphospho-uncarboxylated matrix gla protein (dp-ucMGP) that result from vitamin K deficiency were consistently associated with poor clinical outcomes during COVID-19. Vitamin K-activated matrix gla protein (MGP) is required to protect against elastic fibre degradation, and a deficiency may contribute to pathology. However, intervention trials assessing the effects of vitamin K supplementation in COVID-19 are lacking. Methods: This is a single-centre, phase 2, double-blind, randomised, placebo-controlled trial investigating the effects of vitamin K2 supplementation in 40 hospitalised COVID-19 patients requiring supplemental oxygen. Individuals were randomly assigned in a 1:1 ratio to receive 999 mcg of vitamin K2-menaquinone-7 (MK-7)-or a placebo daily until discharge or for a maximum of 14 days. Dp-ucMGP, the rate of elastic fibre degradation quantified by desmosine, and hepatic vitamin K status quantified by PIVKA-II were measured. Grade 3 and 4 adverse events were collected daily. As an exploratory objective, circulating vitamin K2 levels were measured. Results: Vitamin K2 was well tolerated and did not increase the number of adverse events. A linear mixed model analysis showed that dp-ucMGP and PIVKA-II decreased significantly in subjects that received supplementation compared to the controls (p = 0.008 and p = 0.0017, respectively), reflecting improved vitamin K status. The decrease in dp-ucMGP correlated with higher plasma MK-7 levels (p = 0.015). No significant effect on desmosine was found (p = 0.545). Conclusions: These results demonstrate that vitamin K2 supplementation during COVID-19 is safe and decreases dp-ucMGP. However, the current dose of vitamin K2 failed to show a protective effect against elastic fibre degradation.
Keywords: COVID-19; desmosine; dp-ucMGP; matrix gla protein; menaquinone-7; vitamin K.
Conflict of interest statement
R.J. discloses the application of a patent on vitamin K in COVID-19 that is held by Emphysema Solutions Bv of which R.J. and J.W. are owners. M.P.J.V., J.W., R.J. and A.S.M.D. had a scientific collaboration with Kappa Bioscience A.S., which is a manufacturer of vitamin K2 (MK-7). R.J. and J.M.W.O. are owners of Desmosine.com. H.D., P.A.J., P.Z., E.B.T. and C.K. declare no competing interests.
Figures
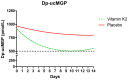

References
-
- Cho J., Lee J., Sia C.H., Koo C.S., Tan B.Y.Q., Hong W., Choi E., Goh X., Chai L., Chandran N.S., et al. Extrapulmonary manifestations and complications of severe acute respiratory syndrome coronavirus-2 infection: A systematic review. Singap. Med. J. 2023;64:349–365. doi: 10.11622/smedj.2021100. - DOI - PMC - PubMed
-
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. - DOI - PMC - PubMed
-
- Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., Hirayama A.V., Mastroiani F., Turtle C.J., Harhay M.O., et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

