Diagnostic Performance of F-18 FDG PET/CT in the Detection of Recurrent Colorectal Cancer: Correlation with Biochemical Markers and Conventional Imaging Modalities
- PMID: 38930131
- PMCID: PMC11204678
- DOI: 10.3390/jcm13123602
Diagnostic Performance of F-18 FDG PET/CT in the Detection of Recurrent Colorectal Cancer: Correlation with Biochemical Markers and Conventional Imaging Modalities
Abstract
Background/Objectives: Although the role of PET/CT imaging is well established in oncology, its diagnostic value in routine monitoring for recurrent colorectal cancer (CRC) is still controversial. The aim was to evaluate the diagnostic value of F-18 FDG PET/CT in detecting recurrent CRC in correlation with CEA, CA 19-9 levels, and conventional imaging modalities (CIM). Methods: Between 2009 and 2023, a retrospective study was performed including 134 CRC patients referred for PET/CT imaging on the suspicion of recurrence, based on elevated CEA and/or CA 19-9 and/or equivocal CIM findings. According to our institution's Tumor Board CRC protocol, after the initial treatment, which was dependent on the TNM stage (neoadjuvant therapy, primary resection, or adjuvant treatment), patients underwent a standard 5-year surveillance including CEA and CA 19-9 measurements, CIM, and colonoscopy, every six months. The statistics, including univariate and multivariate analyses were conducted using the IBM SPSS 20.0 statistical software. p-values < 0.05 were considered statistically significant. Results: Recurrent CRC was confirmed in 54/134 (40.3%) patients with elevated tumor markers. PET/CT showed high diagnostic performance in detecting recurrent CRC with sensitivity, specificity, PPV, NPV, and accuracy of 94.4%, 82.5%, 78.5%, 95.7%, and 87.3%, respectively. The CEA showed a high sensitivity of 98.1% but both low specificity and accuracy of 15% and 48.5%, respectively. The sensitivity, specificity, and accuracy for CA 19-9 and CIM for diagnosis of CRC recurrence were 44.4%, 67.5%, 58.2%, and 51.9%, 98.8%, 79.9%, respectively. The AUC for PET/CT, elevated CEA levels, CIM, and elevated CA 19-9 levels was 0.885 (95% CI: 0.824-0.946; p < 0.001), 0.844 (95% CI: 0.772-0.916; p < 0.001), 0.753 (95% CI: 0.612-0.844; p < 0.001), and 0.547 (95% CI: 0.442-0.652; p = 0.358), respectively. Univariate analysis showed that both PET/CT and CIM positive results were highly associated with CRC recurrence (p < 0.001 and p < 0.001, respectively). At the same time, gender, mucinous tumor type, presence of initial lymph node metastasis (N+), and presence of initial distant metastasis (M+) had no significance (p = 0.211, p = 0.158, p = 0.583, and p = 0.201, respectively). Our multivariate analysis showed that independent predictors for CRC recurrence are positive PET/CT scans (p < 0.001), positive CIM results (p = 0.001), and elevated CA 19-9 levels (p = 0.023). Although CA 19-9 was not detected as a statistically significant predictor in the univariate analysis (p = 0.358), in a multivariate analysis it was recognized as a significant predicting factor in detecting the CRC recurrence (p = 0.023). Conclusions: F-18 FDG PET/CT showed high diagnostic efficacy in CRC recurrence detection, in correlation with CEA levels, CA 19-9 levels, and CIM. This imaging modality should be routinely integrated into the post-operative follow-op in patients with elevated tumor markers.
Keywords: CA 19-9; CEA; F-18 FDG PET/CT; colorectal cancer; conventional imaging; detection; recurrence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

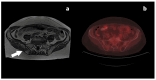
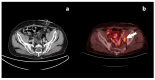
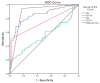
References
-
- Sargent D., Sobrero A., Grothey A., O’Connell M.J., Buyse M., Andre T., Zheng Y., Green E., Labianca R., O’Callaghan C., et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. - DOI - PMC - PubMed
-
- Shah M.A., Renfro L.A., Allegra C.J., Andre T., de Gramont A., Schmoll H.J., Haller D.G., Alberts S.R., Yothers G., Sargent D.J., et al. Impact of Patient Factors on Recurrence Risk and Time Dependency of Oxaliplatin Benefit in Patients with Colon Cancer: Analysis from Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database. J. Clin. Oncol. 2016;34:843–853. doi: 10.1200/JCO.2015.63.0558. - DOI - PMC - PubMed
-
- Nikolic N., Radosavljevic D., Gavrilovic D., Nikolic V., Stanic N., Spasic J., Cecev T., Castellvi-Bel S., Cavic M., Jankovic G. Prognostic factors for post-recurrence survival in stage II and III colorectal carcinoma patients. Medicina. 2021;57:1108. doi: 10.3390/medicina57101108. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

