New-Generation Materials for Hydrogen Storage in Medium-Entropy Alloys
- PMID: 38930265
- PMCID: PMC11205192
- DOI: 10.3390/ma17122897
New-Generation Materials for Hydrogen Storage in Medium-Entropy Alloys
Abstract
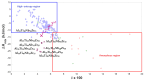
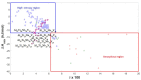
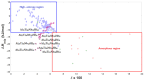
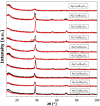
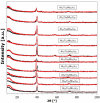
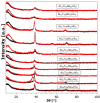
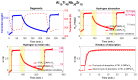
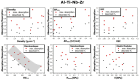
This study presents the design, preparation, and characterization of thirty new medium-entropy alloys (MEAs) in three systems: Al-Ti-Nb-Zr, Al-Ti-Nb-V, and Al-Ti-Nb-Hf. The hardness of the alloys ranged from 320 to 800 HV0.3. Among the alloys studied, Al15Ti40Nb30Zr15 exhibited the highest-reversible hydrogen storage capacity (1.03 wt.%), with an H/M value of 0.68, comparable to LaNi5, but with a reduced density (5.11 g·cm-3) and without rare earth elements. This study further reveals a strong correlation between hardness and hydrogen absorption/desorption; higher hardness is responsible for reduced hydrogen uptake. This finding highlights the interplay between a material's properties and hydrogen storage behavior in MEAs, and has implications for the development of efficient hydrogen storage materials.
Keywords: AlTiNbX; absorption; hydrogen; hydrogen storage; medium-entropy alloys.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Energy Strategy. [(accessed on 1 December 2023)]. Available online: https://energy.ec.europa.eu/topics/energy-strategy_en.
-
- European Commission—HyResource. [(accessed on 1 December 2023)]. Available online: https://research.csiro.au/hyresource/policy/international/european-commi...
-
- European Commission The Hydrogen Strategy for a Climate-Neutral Europe. Dk, Vol. 53, No. 9, pp. 1689–1699. 2015. [(accessed on 10 May 2024)]. Available online: https://energy.ec.europa.eu/system/files/2020-07/hydrogen_strategy_0.pdf/
Grants and funding
- APVV-20-0205/Slovak Research and Development Agency
- APVV-21-0274/Slovak Research and Development Agency
- APVV-21-0396/Slovak Research and Development Agency
- APVV-21-0142/Slovak Research and Development Agency
- 2/0039/22/Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences VEGA
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

