Application of Mesoporous Silicas for Adsorption of Organic and Inorganic Pollutants from Rainwater
- PMID: 38930286
- PMCID: PMC11205702
- DOI: 10.3390/ma17122917
Application of Mesoporous Silicas for Adsorption of Organic and Inorganic Pollutants from Rainwater
Abstract
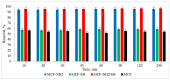
Precipitation is an important factor that influences the quality of surface water in many regions of the world. The pollution of stormwater runoff from roads and parking lots is an understudied area in water quality research. Therefore, a comprehensive analysis of the physicochemical properties of rainwater flowing from parking lots was carried out, considering heavy metals and organic micropollutants. High concentrations of zinc were observed in rainwater, in addition to alkanes, e.g., tetradecane, hexadecane, octadecane, 2,6,10-trimethyldodecane, 2-methyldodecane; phenolic derivatives, such as 2,6-dimethoxyphenol and 2,4-di-tertbutylphenol; and compounds such as benzothiazole. To remove the contaminants present in rainwater, adsorption using silica carriers of the MCF (Mesostructured Cellular Foams) type was performed. Three groups of modified carriers were prepared, i.e., (1) SH (thiol), (2) NH2 (amino), and (3) NH2/SH (amine and thiol functional groups). The research problem, which is addressed in the presented article, is concerned with the silica carrier influence of the functional group on the adsorption efficiency of micropollutants. The study included an evaluation of the effects of adsorption dose and time on the efficiency of the contaminant removal process, as well as an analysis of adsorption isotherms and reaction kinetics. The colour adsorption from rainwater was 94-95% for MCF-NH2 and MCF-NH2/SH. Zinc adsorbance was at a level of 90% for MCF-NH2, and for MCF-NH2/SH, 52%. Studies have shown the high efficacy (100%) of MCF-NH2 in removing organic micropollutants, especially phenolic compounds and benzothiazole. On the other hand, octadecane was the least susceptible to adsorption in each case. It was found that the highest efficiency of removal of organic micropollutants and zinc ions was obtained through the use of functionalized silica NH2.
Keywords: adsorption; functionalized silica; heavy metals; organic compounds; rainwater.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures












References
-
- Gabr M.E., El Shorbagy A.M., Faheem H.B. Assessment of Stormwater Quality in the Context of Traffic Congestion: A Case Study in Egypt. Sustainability. 2023;15:13927. doi: 10.3390/su151813927. - DOI
-
- Generowicz A., Gronba-Chyła A., Kulczycka J., Harazin P., Gaska K., Ciuła J., Ocłoń P. Life Cycle Assessment for the environmental impact assessment of a city’ cleaning system. The case of Cracow (Poland) J. Clean. Prod. 2023;382:135184. doi: 10.1016/j.jclepro.2022.135184. - DOI
-
- Emmanuel E., Balthazard-Accou K., Osnick J. Environmental Management, Sustainable Development and Human Health. CRC Press/Balkema; Boca Raton, FL, USA: 2009. Impact of Urban Wastewater on Biodiversity of Aquatic Ecosystems. Chapter 29.
-
- Gronba-Chyła A., Generowicz A., Kwaśnicki P., Cycoń D., Kwaśny J., Grąz K., Gaska K., Ciuła J. Determining the Effectiveness of Street Cleaning with the Use of Decision Analysis and Research on the Reduction in Chloride in Waste. Energies. 2022;15:3538. doi: 10.3390/en15103538. - DOI
LinkOut - more resources
Full Text Sources

