Scaffold-Hopping Strategies in Aurone Optimization: A Comprehensive Review of Synthetic Procedures and Biological Activities of Nitrogen and Sulfur Analogues
- PMID: 38930878
- PMCID: PMC11206683
- DOI: 10.3390/molecules29122813
Scaffold-Hopping Strategies in Aurone Optimization: A Comprehensive Review of Synthetic Procedures and Biological Activities of Nitrogen and Sulfur Analogues
Abstract
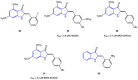
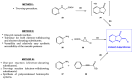
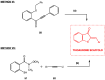
Aurones, particular polyphenolic compounds belonging to the class of minor flavonoids and overlooked for a long time, have gained significative attention in medicinal chemistry in recent years. Indeed, considering their unique and outstanding biological properties, they stand out as an intriguing reservoir of new potential lead compounds in the drug discovery context. Nevertheless, several physicochemical, pharmacokinetic, and pharmacodynamic (P3) issues hinder their progression in more advanced phases of the drug discovery pipeline, making lead optimization campaigns necessary. In this context, scaffold hopping has proven to be a valuable approach in the optimization of natural products. This review provides a comprehensive and updated picture of the scaffold-hopping approaches directed at the optimization of natural and synthetic aurones. In the literature analysis, a particular focus is given to nitrogen and sulfur analogues. For each class presented, general synthetic procedures are summarized, highlighting the key advantages and potential issues. Furthermore, the biological activities of the most representative scaffold-hopped compounds are presented, emphasizing the improvements achieved and the potential for further optimization compared to the aurone class.
Keywords: aurones; azaaurones; benzothiophenone; biological activities; imidazo [1,2-a]pyridine-3-one; indol-3-one; organic synthesis; scaffold hopping; thioaurones.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


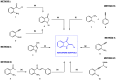
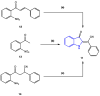
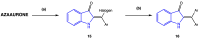
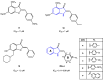






References
-
- Dzobo K. Comprehensive Pharmacology. Volume 2. National Library of Medicine; Bethesda, MD, USA: 2022. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery; pp. 408–422.
-
- Ninomiya M., Koketsu M. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Springer; Berlin/Heidelberg, Germany: 2013. Minor Flavonoids (Chalcones, Flavanones, Dihydrochalcones, and Aurones) pp. 1867–1900.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

