Fucoxanthin Induces Ferroptosis in Cancer Cells via Downregulation of the Nrf2/HO-1/GPX4 Pathway
- PMID: 38930897
- PMCID: PMC11206433
- DOI: 10.3390/molecules29122832
Fucoxanthin Induces Ferroptosis in Cancer Cells via Downregulation of the Nrf2/HO-1/GPX4 Pathway
Abstract
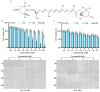
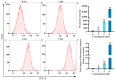
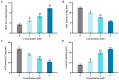
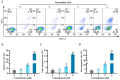
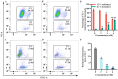
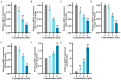
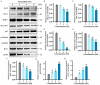
This study investigated the mechanism by which fucoxanthin acts as a novel ferroptosis inducer to inhibit tongue cancer. The MTT assay was used to detect the inhibitory effects of fucoxanthin on SCC-25 human tongue squamous carcinoma cells. The levels of reactive oxygen species (ROS), mitochondrial membrane potential (MMP), glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), and total iron were measured. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and Western blotting were used to assess glutathione peroxidase 4 (GPX4), nuclear factor erythroid 2-related factor 2 (Nrf2), Keap1, solute carrier family 7 member 11 (SLC7A11), transferrin receptor protein 1 (TFR1), p53, and heme oxygenase 1 (HO-1) expression. Molecular docking was performed to validate interactions. Compared with the control group, the activity of fucoxanthin-treated SCC-25 cells significantly decreased in a dose- and time-dependent manner. The levels of MMP, GSH, and SOD significantly decreased in fucoxanthin-treated SCC-25 cells; the levels of ROS, MDA, and total iron significantly increased. mRNA and protein expression levels of Keap1, GPX4, Nrf2, and HO-1 in fucoxanthin-treated cells were significantly decreased, whereas levels of TFR1 and p53 were significantly increased, in a concentration-dependent manner. Molecular docking analysis revealed that binding free energies of fucoxanthin with p53, SLC7A11, GPX4, Nrf2, Keap1, HO-1, and TFR1 were below -5 kcal/mol, primarily based on active site hydrogen bonding. Our findings suggest that fucoxanthin can induce ferroptosis in SCC-25 cells, highlighting its potential as a treatment for tongue cancer.
Keywords: Nrf2/HO−1/GPX4 pathway; anti−cancer; ferroptosis; fucoxanthin.
Conflict of interest statement
The authors declare no conflicts.
Figures

Similar articles
-
[Mechanism of Jiming Powder in inhibiting ferroptosis in treatment of myocardial infarction based on NRF2/HO-1/GPX4 pathway].Zhongguo Zhong Yao Za Zhi. 2025 Jun;50(11):3108-3116. doi: 10.19540/j.cnki.cjcmm.20250122.402. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40686179 Chinese.
-
[Astragalus polysaccharides induces ferroptosis in ovarian adenocarcinoma cells through Nrf2/SLC7A11/GPX4 signaling pathway].Zhongguo Zhong Yao Za Zhi. 2024 Dec;49(23):6459-6467. doi: 10.19540/j.cnki.cjcmm.20240919.501. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 39805792 Chinese.
-
[Mechanism of astragaloside Ⅳ modulation of Nrf2/HO-1/GPX4 pathway to inhibit ferroptosis and ameliorate atherosclerosis in ApoE~(-/-) mice].Zhongguo Zhong Yao Za Zhi. 2024 Jul;49(13):3619-3626. doi: 10.19540/j.cnki.cjcmm.20240321.702. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 39041134 Chinese.
-
[Roles of ferroptosis in the development of diabetic nephropathy].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Dec 25;53(6):708-714. doi: 10.3724/zdxbyxb-2024-0114. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39757741 Free PMC article. Review. Chinese.
-
Therapeutic Effects of Melatonin in the Regulation of Ferroptosis: A Review of Current Evidence.Curr Drug Targets. 2024;25(8):543-557. doi: 10.2174/0113894501284110240426074746. Curr Drug Targets. 2024. PMID: 38706348 Review.
Cited by
-
Static magnetic field promotes the doxorubicin toxicity effects on osteosarcoma cells.Sci Rep. 2025 Apr 7;15(1):11902. doi: 10.1038/s41598-025-96802-0. Sci Rep. 2025. PMID: 40195518 Free PMC article.
-
Targeting ferroptosis: a novel pathway in oral, oropharyngeal, hypopharyngeal, and laryngeal cancers.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 14. doi: 10.1007/s00210-025-04142-7. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40227310 Review.
-
Fucoxanthin Ameliorates Carbon Tetrachloride-Induced Liver Fibrosis in Mice via Nrf2/HO-1/GPX4-Mediated Ferroptosis Pathway.Food Sci Nutr. 2025 Jul 10;13(7):e70589. doi: 10.1002/fsn3.70589. eCollection 2025 Jul. Food Sci Nutr. 2025. PMID: 40654534 Free PMC article.
-
Much More than Nutrients: The Protective Effects of Nutraceuticals on the Blood-Brain Barrier in Diseases.Nutrients. 2025 Feb 21;17(5):766. doi: 10.3390/nu17050766. Nutrients. 2025. PMID: 40077636 Free PMC article. Review.
-
Targeting Ferroptosis in Tumors: Novel Marine-Derived Compounds as Regulators of Lipid Peroxidation and GPX4 Signaling.Mar Drugs. 2025 Jun 19;23(6):258. doi: 10.3390/md23060258. Mar Drugs. 2025. PMID: 40559667 Free PMC article. Review.
References
-
- Obayashi F., Koizumi K., Ito N., Higaki M., Ishida Y., Hamada A., Yamasaki S., Tani R., Yanamoto S. A study of the prognostic factors for late cervical lymph node metastasis and distant metastasis in patients with cT1−2N0 tongue cancer. J. Clin. Med. 2024;13:976. doi: 10.3390/jcm13040976. - DOI - PMC - PubMed
-
- da Silva L.A.B., da Costa L.M., Massetti A.C.P., de Lucena Pereira L., da Silveira E.J.D., Salo T.A., Coletta R.D., da Costa Miguel M.C. Silencing of heat shock factor 1 (HSF1) inhibits proliferation, invasion, and epithelial−mesenchymal transition in oral squamous cell carcinoma. J. Oral Pathol. Med. 2023;52:961–970. doi: 10.1111/jop.13491. - DOI - PubMed
-
- Wu C.Y., Xiang S.Y., Wang H.T., Zhang X.M., Tian X.Y., Tan M.Q., Su W.T. Orally deliverable sequence−targeted fucoxanthin−loaded biomimetic extracellular vesicles for alleviation of nonalcoholic fatty liver disease. ACS Appl. Mater. Interfaces. 2024;16:9854–9867. doi: 10.1021/acsami.3c18029. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

