Modulation of the Human Erythrocyte Antioxidant System by the 5- and 6-Membered Heterocycle-Based Nitroxides
- PMID: 38931005
- PMCID: PMC11207074
- DOI: 10.3390/molecules29122941
Modulation of the Human Erythrocyte Antioxidant System by the 5- and 6-Membered Heterocycle-Based Nitroxides
Abstract
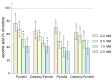
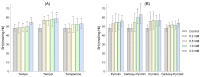
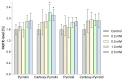
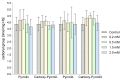
Nitroxides are stable radicals consisting of a nitroxyl group, >N-O•, which carries an unpaired electron. This group is responsible for the paramagnetic and antioxidant properties of these compounds. A recent study evaluated the effects of pyrrolidine and pyrroline derivatives of nitroxides on the antioxidant system of human red blood cells (RBCs). It showed that nitroxides caused an increase in the activity of superoxide dismutase (SOD) and the level of methemoglobin (MetHb) in cells (in pyrroline derivatives) but had no effect on the activity of catalase and lactate dehydrogenase. Nitroxides also reduced the concentration of ascorbic acid (AA) in cells but did not cause any oxidation of proteins or lipids. Interestingly, nitroxides initiated an increase in thiols in the plasma membranes and hemolysate. However, the study also revealed that nitroxides may have pro-oxidant properties. The drop in the AA concentration and the increase in the MetHb level and in SOD activity may indicate the pro-oxidant properties of nitroxides in red blood cells.
Keywords: catalase; lactate dehydrogenase; nitroxide; oxidative stress; red blood cells; superoxide dismutase.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures







References
-
- Ouari O. Nitroxides: Synthesis, Properties and Applications. 1st ed. Royal Society of Chemistry; Cambridge, UK: 2021.
-
- Berliner L.J., editor. Spin Labeling: Theory and Applications. Academic Press; New York, NY, USA: London, UK: 1976.
-
- Rozantsev E., editor. Free Nitroxyl Radicals. Springer; New York, NY, USA: 2013.
-
- Berliner L.J., editor. Spin Labeling 2: Theory and Applications. Academic Press; New York, NY, USA: London, UK: 1979.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

