A Zn(II) Coordination Polymer for Fluorescent Turn-Off Selective Sensing of Heavy Metal Cation and Toxic Inorganic Anions
- PMID: 38931007
- PMCID: PMC11206703
- DOI: 10.3390/molecules29122943
A Zn(II) Coordination Polymer for Fluorescent Turn-Off Selective Sensing of Heavy Metal Cation and Toxic Inorganic Anions
Abstract
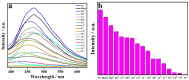
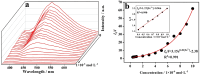
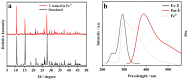
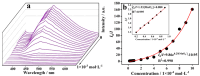
A novel coordination polymer [Zn(atyha)2]n (1) (Hatyha = 2-(2-aminothiazole-4-yl)-2- hydroxyiminoacetic acid) was constructed by hydrothermal reaction of Zn2+ with Hatyha ligand. CP 1 exhibits a 2D (4,4)-connected topological framework with Schläfli symbol of {44·62}, where atyha- anions serve as tridentate ligands, bridging with Zn2+ through carboxylate, thiazole and oxime groups. CP 1 displays a strong ligand-based photoluminescence at 390 nm in the solid state, and remains significantly structurally stable in water. Interestingly, it can be utilized as a fluorescent probe for selective and sensitive sensing of Fe3+, Cr2O72- and MnO4- through the fluorescent turn-off effect with limit of detection (LOD) of 3.66 × 10-6, 2.38 × 10-5 and 2.94 × 10-6 M, respectively. Moreover, the efficient recyclability for detection of Fe3+ and Cr2O72- is better than that for MnO4-. The mechanisms of fluorescent quenching involve reversible overlap of UV-Vis absorption bands of the analytes (Fe3+, Cr2O72- and MnO4-) with fluorescence excitation and emission bands for CP 1, respectively.
Keywords: coordination polymer; fluorescent; inorganic anion; metal cation; selective sensing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Zhao X.Y., Wu J.F., Tian W. Terbium(III)-based coordination polymer with millimeter-size single crystals and high selectivity and sensitivity for folic acid. CrystEngComm. 2023;25:945–952. doi: 10.1039/D2CE01608G. - DOI
-
- Liu L.J., Zhu H., Han C., Cui G.H., Fu L.S. Luminescent detecting of Fe3+ and Cr2O72− ions by three ternary 2D coordination polymers. Polyhedron. 2021;198:115074. doi: 10.1016/j.poly.2021.115074. - DOI
-
- Barnett B.R., Gonzalez M.I., Long J.R. Recent progress towards light hydrocarbon separations using metal-organic frameworks. Trends Chem. 2019;1:159–171. doi: 10.1016/j.trechm.2019.02.012. - DOI
-
- Zhao Q., Li Q.Y., Li J. Structures, fluorescence and magnetism of a series of coordination polymers driven by a tricarboxypyridine ligand. CrystEngComm. 2022;24:6751–6761. doi: 10.1039/D2CE00726F. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

