Structure-Based Design, Virtual Screening, and Discovery of Novel Patulin Derivatives as Biogenic Photosystem II Inhibiting Herbicides
- PMID: 38931142
- PMCID: PMC11207439
- DOI: 10.3390/plants13121710
Structure-Based Design, Virtual Screening, and Discovery of Novel Patulin Derivatives as Biogenic Photosystem II Inhibiting Herbicides
Abstract
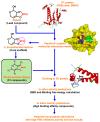
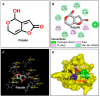
Computer-aided design usually gives inspirations and has become a vital strategy to develop novel pesticides through reconstructing natural lead compounds. Patulin, an unsaturated heterocyclic lactone mycotoxin, is a new natural PSII inhibitor and shows significant herbicidal activity to various weeds. However, some evidence, especially the health concern, prevents it from developing as a bioherbicide. In this work, molecular docking and toxicity risk prediction are combined to construct interaction models between the ligand and acceptor, and design and screen novel derivatives. Based on the analysis of a constructed patulin-Arabidopsis D1 protein docking model, in total, 81 derivatives are designed and ranked according to quantitative estimates of drug-likeness (QED) values and free energies. Among the newly designed derivatives, forty-five derivatives with better affinities than patulin are screened to further evaluate their toxicology. Finally, it is indicated that four patulin derivatives, D3, D6, D34, and D67, with higher binding affinity but lower toxicity than patulin have a great potential to develop as new herbicides with improved potency.
Keywords: D1 protein; docking; homology modeling; natural product; photosynthetic inhibitor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Structure-based ligand design and discovery of novel tenuazonic acid derivatives with high herbicidal activity.J Adv Res. 2022 Sep;40:29-44. doi: 10.1016/j.jare.2021.12.001. Epub 2021 Dec 14. J Adv Res. 2022. PMID: 36100332 Free PMC article.
-
Action Mode of the Mycotoxin Patulin as a Novel Natural Photosystem II Inhibitor.J Agric Food Chem. 2021 Jul 7;69(26):7313-7323. doi: 10.1021/acs.jafc.1c01811. Epub 2021 Jun 24. J Agric Food Chem. 2021. PMID: 34165302
-
Action of the fungal compound citrinin, a bioherbicide candidate, on photosystem II.Pest Manag Sci. 2024 Jan;80(1):133-148. doi: 10.1002/ps.7513. Epub 2023 May 11. Pest Manag Sci. 2024. PMID: 37103431
-
Recent advances in tenuazonic acid as a potential herbicide.Pestic Biochem Physiol. 2017 Nov;143:252-257. doi: 10.1016/j.pestbp.2017.01.003. Epub 2017 Jan 4. Pestic Biochem Physiol. 2017. PMID: 29183600 Review.
-
Importance of molecular computer modeling in anticancer drug development.J BUON. 2007 Sep;12 Suppl 1:S101-18. J BUON. 2007. PMID: 17935268 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

