Modeling of Intracellular Taurine Levels Associated with Ovarian Cancer Reveals Activation of p53, ERK, mTOR and DNA-Damage-Sensing-Dependent Cell Protection
- PMID: 38931171
- PMCID: PMC11206249
- DOI: 10.3390/nu16121816
Modeling of Intracellular Taurine Levels Associated with Ovarian Cancer Reveals Activation of p53, ERK, mTOR and DNA-Damage-Sensing-Dependent Cell Protection
Abstract
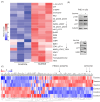
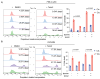
Taurine, a non-proteogenic amino acid and commonly used nutritional supplement, can protect various tissues from degeneration associated with the action of the DNA-damaging chemotherapeutic agent cisplatin. Whether and how taurine protects human ovarian cancer (OC) cells from DNA damage caused by cisplatin is not well understood. We found that OC ascites-derived cells contained significantly more intracellular taurine than cell culture-modeled OC. In culture, elevation of intracellular taurine concentration to OC ascites-cell-associated levels suppressed proliferation of various OC cell lines and patient-derived organoids, reduced glycolysis, and induced cell protection from cisplatin. Taurine cell protection was associated with decreased DNA damage in response to cisplatin. A combination of RNA sequencing, reverse-phase protein arrays, live-cell microscopy, flow cytometry, and biochemical validation experiments provided evidence for taurine-mediated induction of mutant or wild-type p53 binding to DNA, activation of p53 effectors involved in negative regulation of the cell cycle (p21), and glycolysis (TIGAR). Paradoxically, taurine's suppression of cell proliferation was associated with activation of pro-mitogenic signal transduction including ERK, mTOR, and increased mRNA expression of major DNA damage-sensing molecules such as DNAPK, ATM and ATR. While inhibition of ERK or p53 did not interfere with taurine's ability to protect cells from cisplatin, suppression of mTOR with Torin2, a clinically relevant inhibitor that also targets DNAPK and ATM/ATR, broke taurine's cell protection. Our studies implicate that elevation of intracellular taurine could suppress cell growth and metabolism, and activate cell protective mechanisms involving mTOR and DNA damage-sensing signal transduction.
Keywords: cell protection; fallopian tube cells; genotoxic stress; growth suppression; ovarian cancer cells; signal transduction; taurine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









Update of
-
Modeling of Intracellular Taurine Levels Associated with Ovarian Cancer Reveals Activation of p53, ERK, mTOR and DNA-damage-sensing-dependent Cell Protection.bioRxiv [Preprint]. 2024 May 14:2023.02.24.529893. doi: 10.1101/2023.02.24.529893. bioRxiv. 2024. Update in: Nutrients. 2024 Jun 09;16(12):1816. doi: 10.3390/nu16121816. PMID: 36909636 Free PMC article. Updated. Preprint.
References
-
- Ansar M., Ranza E., Shetty M., Paracha S.A., Azam M., Kern I., Iwaszkiewicz J., Farooq O., Pournaras C.J., Malcles A., et al. Taurine treatment of retinal degeneration and cardiomyopathy in a consanguineous family with SLC6A6 taurine transporter deficiency. Hum. Mol. Genet. 2020;29:618–623. doi: 10.1093/hmg/ddz303. - DOI - PMC - PubMed
-
- Kim K.S., Oh D.H., Kim J.Y., Lee B.G., You J.S., Chang K.J., Chung H.J., Yoo M.C., Yang H.I., Kang J.H., et al. Taurine ameliorates hyperglycemia and dyslipidemia by reducing insulin resistance and leptin level in Otsuka Long-Evans Tokushima fatty (OLETF) rats with long-term diabetes. Exp. Mol. Med. 2012;44:665–673. doi: 10.3858/emm.2012.44.11.075. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

