A Cross-Sectional Validation Study of Camry EH101 versus JAMAR Plus Handheld Dynamometers in Colorectal Cancer Patients and Their Correlations with Bioelectrical Impedance and Nutritional Status
- PMID: 38931179
- PMCID: PMC11206484
- DOI: 10.3390/nu16121824
A Cross-Sectional Validation Study of Camry EH101 versus JAMAR Plus Handheld Dynamometers in Colorectal Cancer Patients and Their Correlations with Bioelectrical Impedance and Nutritional Status
Abstract
Background: Reduced muscle strength (dynapenia) and mass (atrophy) are prognostic factors in oncology. Measuring maximal handgrip strength with dynamometers is feasible but limited by the cost of the reference device (JAMAR).
Methods: A cross-sectional study was conducted on colorectal cancer outpatients treated with chemotherapy or under active surveillance in our center from September 2022 to July 2023. Accuracy, reliability, and concordance were compared for two handheld dynamometers: the JAMAR Plus (the gold-standard device) and the Camry EH101 (a low-cost index device). A simultaneous nutritional diagnosis with GLIM criteria and bioelectrical impedance analysis (BIA) was carried out.
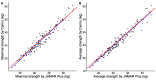
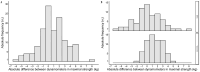
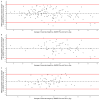
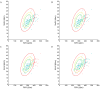
Results: A total of 134 participants were included. The median of maximal strength for the JAMAR Plus had a non-significant difference of 1.4 kg from the Camry EH101. The accuracy and reliability of the devices were high. Bland-Altman analysis showed a 0.8 kg bias and -4.1 to 5.6 kg limits of agreement (LoA); a 0.1 kg bias and -5.3 to 5.4 kg LoA in men; a 1.5 kg bias and -2.2 to 5.3 kg LoA in women. In total, 29.85% of the participants were malnourished. Prevalence of dynapenia increased from 3.67% with the JAMAR Plus to 5.14% with the Camry EH101. Both devices had a moderate and significant correlation with BIA-estimated muscle mass.
Conclusions: The Camry EH101 was a cost-effective alternative to JAMAR Plus in our sample.
Keywords: Camry; GLIM criteria; JAMAR; Smedley; bioelectrical impedance; colorectal cancer; dynamometer; handgrip strength; morphofunctional assessment; muscle mass.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- van Baar H., Winkels R.M., Brouwer J.G.M., Posthuma L., Bours M.J.L., Weijenberg M.P., Boshuizen H.C., van Zutphen M., van Duijnhoven F.J.B., Kok D.E., et al. Associations of Abdominal Skeletal Muscle Mass, Fat Mass, and Mortality among Men and Women with Stage I-III Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2020;29:956–965. doi: 10.1158/1055-9965.EPI-19-1134. - DOI - PubMed
-
- Shirdel M., Andersson F., Myte R., Axelsson J., Rutegård M., Blomqvist L., Riklund K., van Guelpen B., Palmqvist R., Gylling B. Body Composition Measured by Computed Tomography Is Associated with Colorectal Cancer Survival, Also in Early-Stage Disease. Acta Oncol. 2020;59:799–808. doi: 10.1080/0284186X.2020.1744716. - DOI - PubMed
-
- Derksen J.W.G., Kurk S.A., Peeters P.H.M., Dorresteijn B., Jourdan M., van der Velden A.M.T., Nieboer P., de Jong R.S., Honkoop A.H., Punt C.J.A., et al. The Association between Changes in Muscle Mass and Quality of Life in Patients with Metastatic Colorectal Cancer. J. Cachexia Sarcopenia Muscle. 2020;11:919–928. doi: 10.1002/jcsm.12562. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

