Efficacy and Safety of Panax ginseng Sprout Extract in Subjective Memory Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
- PMID: 38931306
- PMCID: PMC11206504
- DOI: 10.3390/nu16121952
Efficacy and Safety of Panax ginseng Sprout Extract in Subjective Memory Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
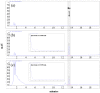
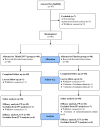
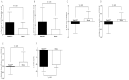
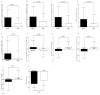
Sprout ginseng extract (ThinkGIN™) manufactured through a smart farm system has been shown to improve memory in preclinical studies. This study conducted a 12-week randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of ThinkGIN™ for improving memory in subjective memory impairment (SMI). Subjects aged 55 to 75 years with SMI participated in this study. A total of 80 subjects who met the inclusion/exclusion criteria were assigned to the ThinkGIN™ group (n = 40, 450 mg ThinkGIN™/day) or a placebo group (n = 40). Efficacy and safety evaluations were conducted before intervention and at 12 weeks after intervention. As a result of 12 weeks of ThinkGIN™ intake, significant differences in SVLT, RCFT, MoCA-K, PSQI-K, and AChE were observed between the two groups. Safety evaluation (AEs, laboratory tests, vital signs, and electrocardiogram) revealed that ThinkGIN™ was safe with no clinically significant changes. Therefore, ThinkGIN™ has the potential to be used as a functional food to improve memory.
Keywords: clinical trial; functional food; memory improvement; smart farming; sprout ginseng; subjective memory impairment.
Conflict of interest statement
Authors Ki-Chan Ha and Yu-Kyung Park were employed by the Healthcare Claims & Management Inc. Author Tae-Young Kim is employed by BTC Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures
Similar articles
-
Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a double-blind, randomized, cross-over clinical trial.Eur J Nutr. 2019 Apr;58(3):1237-1245. doi: 10.1007/s00394-018-1642-0. Epub 2018 Feb 24. Eur J Nutr. 2019. PMID: 29478187 Clinical Trial.
-
Safety and tolerability of Panax ginseng root extract: a randomized, placebo-controlled, clinical trial in healthy Korean volunteers.J Altern Complement Med. 2012 Nov;18(11):1061-9. doi: 10.1089/acm.2011.0591. Epub 2012 Aug 21. J Altern Complement Med. 2012. PMID: 22909282 Free PMC article. Clinical Trial.
-
Antifatigue effects of Panax ginseng C.A. Meyer: a randomised, double-blind, placebo-controlled trial.PLoS One. 2013 Apr 17;8(4):e61271. doi: 10.1371/journal.pone.0061271. Print 2013. PLoS One. 2013. PMID: 23613825 Free PMC article. Clinical Trial.
-
The efficacy of ginseng. A systematic review of randomised clinical trials.Eur J Clin Pharmacol. 1999 Oct;55(8):567-75. doi: 10.1007/s002280050674. Eur J Clin Pharmacol. 1999. PMID: 10541774
-
A systematic review of research investigating the physiological and psychological effects of combining Ginkgo biloba and Panax ginseng into a single treatment in humans: Implications for research design and analysis.Brain Behav. 2019 Mar;9(3):e01217. doi: 10.1002/brb3.1217. Epub 2019 Feb 6. Brain Behav. 2019. PMID: 30729756 Free PMC article.
Cited by
-
Panax ginseng: A modulator of amyloid, tau pathology, and cognitive function in Alzheimer's disease.J Ginseng Res. 2025 Jul;49(4):348-355. doi: 10.1016/j.jgr.2025.03.011. Epub 2025 Apr 2. J Ginseng Res. 2025. PMID: 40621081 Free PMC article. Review.
-
Natural Products and Their Neuroprotective Effects in Degenerative Brain Diseases: A Comprehensive Review.Int J Mol Sci. 2024 Oct 18;25(20):11223. doi: 10.3390/ijms252011223. Int J Mol Sci. 2024. PMID: 39457003 Free PMC article. Review.
References
-
- United Nations . World Population Prospects 2019: Highlights. United Nations; New York, NY, USA: 2019. Department of Economic and Social Affairs, Population Division.
-
- Chiu C.-C., Su K.-P., Cheng T.-C., Liu H.-C., Chang C.-J., Dewey M.E., Stewart R., Huang S.-Y. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1538–1544. doi: 10.1016/j.pnpbp.2008.05.015. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

