Taurine Neuroprotection and Neurogenesis Effect in Chronic Ethanol-Induced Rats
- PMID: 38931326
- PMCID: PMC11206532
- DOI: 10.3390/nu16121973
Taurine Neuroprotection and Neurogenesis Effect in Chronic Ethanol-Induced Rats
Abstract
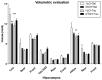
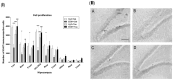
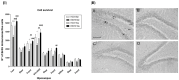
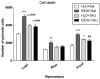
Taurine (2-aminoethanesulfonic acid) is a non-protein β-amino acid essential for cellular homeostasis, with antioxidant, anti-inflammatory, and cytoprotective properties that are crucial for life maintenance. This study aimed to evaluate the effects of taurine administration on hippocampal neurogenesis, neuronal preservation, or reverse damage in rats exposed to forced ethanol consumption in an animal model. Wistar rats were treated with ethanol (EtOH) for a 28-day period (5% in the 1st week, 10% in the 2nd week, and 20% in the 3rd and 4th weeks). Two taurine treatment protocols (300 mg/kg i.p.) were implemented: one during ethanol consumption to analyze neuroprotection, and another after ethanol consumption to assess the reversal of ethanol-induced damage. Overall, the results demonstrated that taurine treatment was effective in protecting against deficits induced by ethanol consumption in the dentate gyrus. The EtOH+TAU group showed a significant increase in cell proliferation (145.8%) and cell survival (54.0%) compared to the EtOH+Sal group. The results also indicated similar effects regarding the reversal of ethanol-induced damage 28 days after the cessation of ethanol consumption. The EtOH+TAU group exhibited a significant increase (41.3%) in the number of DCX-immunoreactive cells compared to the EtOH+Sal group. However, this amino acid did not induce neurogenesis in the tissues of healthy rats, implying that its activity may be contingent upon post-injury stimuli.
Keywords: ethanol consumption; hippocampus; neurogenesis; neuroprotection; taurine.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- The Earliest Alcoholic Beverage in the World|Research-Penn Museum. [(accessed on 13 June 2024)]. Available online: https://www.penn.museum/research/project.php?pid=12.
-
- Nielsen B., Andersen K. Alcohol, anxiety, and depression. Ugeskr. Laeger. 2022;184:V10210816. - PubMed
-
- Llamosas-Falcón L., Rehm J., Bright S., Buckley C., Carr T., Kilian C., Lasserre A.M., Lemp J.M., Zhu Y., Probst C. The Relationship Between Alcohol Consumption, BMI, and Type 2 Diabetes: A Systematic Review and Dose-Response Meta-Analysis. Diabetes Care. 2023;46:2076–2083. doi: 10.2337/dc23-1015. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

