Synovial Membrane Is a Major Producer of Extracellular Inorganic Pyrophosphate in Response to Hypoxia
- PMID: 38931405
- PMCID: PMC11206467
- DOI: 10.3390/ph17060738
Synovial Membrane Is a Major Producer of Extracellular Inorganic Pyrophosphate in Response to Hypoxia
Abstract
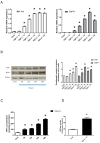
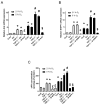
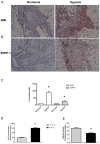
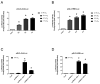
Calcium pyrophosphate dehydrate (CPPD) crystals are found in the synovial fluid of patients with articular chondrocalcinosis or sometimes with osteoarthritis. In inflammatory conditions, the synovial membrane (SM) is subjected to transient hypoxia, especially during movement. CPPD formation is supported by an increase in extracellular inorganic pyrophosphate (ePPi) levels, which are mainly controlled by the transporter Ank and ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1). We demonstrated previously that transforming growth factor (TGF)-β1 increased ePPi production by inducing Ank and Enpp1 expression in chondrocytes. As the TGF-β1 level raises in synovial fluid under hypoxic conditions, we investigated whether hypoxia may transform SM as a major source of ePPi production. Synovial fibroblasts and SM explants were exposed to 10 ng/mL of TGF-β1 in normoxic or hypoxic (5% O2) culture conditions. Ank and Enpp1 expression were assessed by quantitative PCR, Western blot and immunohistochemistry. ePPi was quantified in culture supernatants. RNA silencing was used to define the respective roles of Ank and Enpp1 in TGF-β1-induced ePPi generation. The molecular mechanisms involved in hypoxia were investigated using an Ank promoter reporter plasmid for transactivation studies, as well as gene overexpression and RNA silencing, the respective role of hypoxia-induced factor (HIF)-1 and HIF-2. Our results showed that TGF-β1 increased Ank, Enpp1, and therefore ePPi production in synovial fibroblasts and SM explants. Ank was the major contributor in ePPi production compared to ENPP1. Hypoxia increased ePPi levels on its own and enhanced the stimulating effect of TGF-β1. Hypoxic conditions enhanced Ank promoter transactivation in an HIF-1-dependent/HIF-2-independent fashion. We demonstrated that under hypoxia, SM is an important contributor to ePPi production in the joint through the induction of Enpp1 and Ank. These findings are of interest as a rationale for the beneficial effect of anti-inflammatory drugs on SM in crystal depositions.
Keywords: Ank; ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1); extracellular inorganic pyrophosphate (ePPi); hypoxia; synovial fibroblasts.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
Similar articles
-
Inorganic pyrophosphate generation by transforming growth factor-beta-1 is mainly dependent on ANK induction by Ras/Raf-1/extracellular signal-regulated kinase pathways in chondrocytes.Arthritis Res Ther. 2007;9(6):R122. doi: 10.1186/ar2330. Arthritis Res Ther. 2007. PMID: 18034874 Free PMC article.
-
Up-regulated expression of cartilage intermediate-layer protein and ANK in articular hyaline cartilage from patients with calcium pyrophosphate dihydrate crystal deposition disease.Arthritis Rheum. 2002 Dec;46(12):3218-29. doi: 10.1002/art.10632. Arthritis Rheum. 2002. PMID: 12483726
-
Histone Deacetylase Inhibitors Downregulate Calcium Pyrophosphate Crystal Formation in Human Articular Chondrocytes.Int J Mol Sci. 2022 Feb 26;23(5):2604. doi: 10.3390/ijms23052604. Int J Mol Sci. 2022. PMID: 35269745 Free PMC article.
-
Modulation of chondrocyte production of extracellular inorganic pyrophosphate.Curr Opin Rheumatol. 2004 May;16(3):268-72. doi: 10.1097/00002281-200405000-00017. Curr Opin Rheumatol. 2004. PMID: 15103256 Review.
-
Extracellular pyrophosphate: The body's "water softener".Bone. 2020 May;134:115243. doi: 10.1016/j.bone.2020.115243. Epub 2020 Jan 16. Bone. 2020. PMID: 31954851 Review.
Cited by
-
Genome-wide association study in chondrocalcinosis reveals ENPP1 as a candidate therapeutic target in calcium pyrophosphate deposition disease.Ann Rheum Dis. 2025 Jun;84(6):1023-1032. doi: 10.1016/j.ard.2025.04.002. Epub 2025 May 28. Ann Rheum Dis. 2025. PMID: 40483170
References
-
- Abhishek A., Doherty S., Maciewicz R., Muir K., Zhang W., Doherty M. Evidence of a Systemic Predisposition to Chondrocalcinosis and Association Between Chondrocalcinosis and Osteoarthritis at Distant Joints: A Cross-Sectional Study: Systemic Predisposition to CC and Association of CC and OA. Arthritis Care Res. 2013;65:1052–1058. doi: 10.1002/acr.21952. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

