Dichloroacetate for Cancer Treatment: Some Facts and Many Doubts
- PMID: 38931411
- PMCID: PMC11206832
- DOI: 10.3390/ph17060744
Dichloroacetate for Cancer Treatment: Some Facts and Many Doubts
Abstract
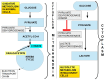
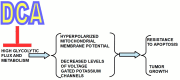
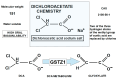
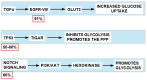
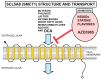
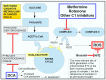
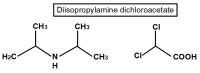
Rarely has a chemical elicited as much controversy as dichloroacetate (DCA). DCA was initially considered a dangerous toxic industrial waste product, then a potential treatment for lactic acidosis. However, the main controversies started in 2008 when DCA was found to have anti-cancer effects on experimental animals. These publications showed contradictory results in vivo and in vitro such that a thorough consideration of this compound's in cancer is merited. Despite 50 years of experimentation, DCA's future in therapeutics is uncertain. Without adequate clinical trials and health authorities' approval, DCA has been introduced in off-label cancer treatments in alternative medicine clinics in Canada, Germany, and other European countries. The lack of well-planned clinical trials and its use by people without medical training has discouraged consideration by the scientific community. There are few thorough clinical studies of DCA, and many publications are individual case reports. Case reports of DCA's benefits against cancer have been increasing recently. Furthermore, it has been shown that DCA synergizes with conventional treatments and other repurposable drugs. Beyond the classic DCA target, pyruvate dehydrogenase kinase, new target molecules have also been recently discovered. These findings have renewed interest in DCA. This paper explores whether existing evidence justifies further research on DCA for cancer treatment and it explores the role DCA may play in it.
Keywords: cancer; dichloroacetate; glioma; lactic acidosis; pyruvate dehydrogenase; pyruvate dehydrogenase kinase; pyruvate dehydrogenase phosphatase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
The Importance of Gender-Related Anticancer Research on Mitochondrial Regulator Sodium Dichloroacetate in Preclinical Studies In Vivo.Cancers (Basel). 2019 Aug 20;11(8):1210. doi: 10.3390/cancers11081210. Cancers (Basel). 2019. PMID: 31434295 Free PMC article. Review.
-
Dichloroacetate and Pyruvate Metabolism: Pyruvate Dehydrogenase Kinases as Targets Worth Investigating for Effective Therapy of Toxoplasmosis.mSphere. 2021 Jan 6;6(1):e01002-20. doi: 10.1128/mSphere.01002-20. mSphere. 2021. PMID: 33408226 Free PMC article.
-
Dichloroacetate is an antimetabolite that antagonizes acetate and deprives cancer cells from its benefits: A novel evidence-based medical hypothesis.Med Hypotheses. 2019 Jan;122:206-209. doi: 10.1016/j.mehy.2018.11.012. Epub 2018 Nov 22. Med Hypotheses. 2019. PMID: 30593413
-
[Dose dependent effects of dichloroacetate on lactic acidosis in dogs].Masui. 1989 Aug;38(8):1005-11. Masui. 1989. PMID: 2810694 Japanese.
-
[Sodium dichloroacetate--a substance with manifold therapeutic potential].Wien Klin Wochenschr. 1990 Feb 2;102(3):65-9. Wien Klin Wochenschr. 1990. PMID: 2180210 Review. German.
Cited by
-
Drug Repurposing: A Conduit to Unravelling Metabolic Reprogramming for Cancer Treatment.Mini Rev Med Chem. 2025;25(8):601-627. doi: 10.2174/0113895575339660250106093738. Mini Rev Med Chem. 2025. PMID: 39835565 Review.
-
Glucose Metabolism and Tumor Microenvironment: Mechanistic Insights and Therapeutic Implications.Int J Mol Sci. 2025 Feb 22;26(5):1879. doi: 10.3390/ijms26051879. Int J Mol Sci. 2025. PMID: 40076506 Free PMC article. Review.
-
Emerging Role of Extracellular pH in Tumor Microenvironment as a Therapeutic Target for Cancer Immunotherapy.Cells. 2024 Nov 20;13(22):1924. doi: 10.3390/cells13221924. Cells. 2024. PMID: 39594672 Free PMC article. Review.
-
Investigation of the protective effects of dichloroacetic acid in a rat model of diabetic neuropathy.BMC Pharmacol Toxicol. 2025 Jan 22;26(1):15. doi: 10.1186/s40360-025-00849-8. BMC Pharmacol Toxicol. 2025. PMID: 39844306 Free PMC article.
-
Unlocking the Role of Metabolic Pathways in Brain Metastatic Disease.Cells. 2025 May 13;14(10):707. doi: 10.3390/cells14100707. Cells. 2025. PMID: 40422210 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

