Novel Anti-Enterovirus A71 Compounds Discovered by Repositioning Antivirals from the Open-Source MMV Pandemic Response Box
- PMID: 38931452
- PMCID: PMC11206571
- DOI: 10.3390/ph17060785
Novel Anti-Enterovirus A71 Compounds Discovered by Repositioning Antivirals from the Open-Source MMV Pandemic Response Box
Abstract
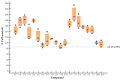
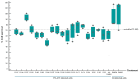
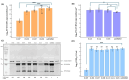
The open-source drug library, namely, MMV Pandemic Response Box, contains 153 antiviral agents, a chemically and pharmacologically diverse mixture of early-stage, emerging anti-infective scaffolds, and mature compounds currently undergoing clinical development. Hence, the Pandemic Response Box might contain compounds that bind and interfere with target molecules or cellular pathways that are conserved or shared among the closely related viruses with enterovirus A71 (EV-A71). This study aimed to screen antiviral agents included in the Pandemic Response Box for repurposing to anti-EV-A71 activity and investigate the inhibitory effects of the compounds on viral replication. The compounds' cytotoxicity and ability to rescue infected cells were determined by % cell survival using an SRB assay. The hit compounds were verified for anti-EV-A71 activity by virus reduction assays for viral RNA copy numbers, viral protein synthesis, and mature particle production using qRT-PCR, Western blot analysis, and CCID50 assay, respectively. It was found that some of the hit compounds could reduce EV-A71 genome replication and protein synthesis. D-D7 (2-pyridone-containing human rhinovirus 3C protease inhibitor) exhibited the highest anti-EV-A71 activity. Even though D-D7 has been originally indicated as a polyprotein processing inhibitor of human rhinovirus 3C protease, it could be repurposed as an anti-EV-A71 agent.
Keywords: antivirus drugs; enterovirus; enterovirus A71; hand-foot-mouth disease; repurposed drugs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Antiviral effect in association with anti-apoptosis and anti-autophagy of repurposing formoterol fumarate dihydrate on enterovirus A71-infected neuronal cells.Virus Res. 2022 Apr 2;311:198692. doi: 10.1016/j.virusres.2022.198692. Epub 2022 Jan 29. Virus Res. 2022. PMID: 35093474
-
Antiviral activity of silymarin in comparison with baicalein against EV-A71.BMC Complement Med Ther. 2020 Mar 23;20(1):97. doi: 10.1186/s12906-020-2880-2. BMC Complement Med Ther. 2020. PMID: 32293397 Free PMC article.
-
Drug repurposing of pyrimidine analogs as potent antiviral compounds against human enterovirus A71 infection with potential clinical applications.Sci Rep. 2020 May 18;10(1):8159. doi: 10.1038/s41598-020-65152-4. Sci Rep. 2020. PMID: 32424333 Free PMC article.
-
Recent Advances in Enterovirus A71 Infection and Antiviral Agents.Lab Invest. 2024 Feb;104(2):100298. doi: 10.1016/j.labinv.2023.100298. Epub 2023 Nov 25. Lab Invest. 2024. PMID: 38008182 Review.
-
[Progress in Research on Structure, Function and Antiviral of Enterovirus A71 3C Protein].Bing Du Xue Bao. 2015 Jul;31(4):468-73. Bing Du Xue Bao. 2015. PMID: 26524922 Review. Chinese.
References
Grants and funding
LinkOut - more resources
Full Text Sources

