In Vitro/In Vivo Correlation of Two Extended-Release Cilostazol Formulations
- PMID: 38931454
- PMCID: PMC11206399
- DOI: 10.3390/ph17060787
In Vitro/In Vivo Correlation of Two Extended-Release Cilostazol Formulations
Abstract
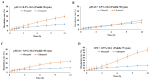
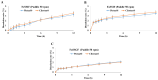
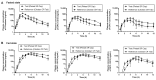
This study aims to evaluate and determine the correlation between in vitro release and in vivo pharmacokinetics of two extended-release dosage forms of Cilostazol. In vitro release profiles for two dosage forms, tablet and capsule, were analyzed under physiologically mimicked medium conditions using the paddle and basket USP release apparatus. A single-dose, two-period crossover study design in beagle dogs was applied for the pharmacokinetic study. The fed and fast effects were considered for evaluation. Pseudo gastric release medium transfer setup study from pH 1.2 to pH 6.8 (+0.5% SLS) and pH 1.2 to pH 6.8 (+1.0% SLS) demonstrated that Pletaal® SR 200 mg capsules have higher drug release rates than Cilostan® CR 200 mg tablets. Similarly, in vivo study showed Cilostazol concentration in plasma and AUC was lower under the fast state than the fed state. The ratio of least squared geometric mean values, Cmax, AUC0-t, and AUC0-inf of Cilostazol were 2.53-fold, 2.89-fold, and 2.87-fold higher for Pletaal® SR 200 mg capsules compared with Cilostan® CR 200 mg tablets, respectively. Correlation of in vitro/in vivo data indicated that Pletal® SR 200 mg capsules have better release and pharmacodynamic effect than Cilostan® CR 200 mg tablets.
Keywords: capsule; cilostazol; in vitro; in vivo; pharmacokinetics; release; two-period crossover design.
Conflict of interest statement
Young-Joon Park is a current IMDpharm Inc. Employee. The company had a role in the design of the study but did not have a role in the collection, analysis, or interpretation of data, writing of the manuscript, or decision to publish the results.
Figures
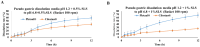


Similar articles
-
Pharmacokinetic study of two extended-release formulations of cilostazol in healthy Korean subjects: A randomized, open-label, multiple-dose, two-period crossover study .Int J Clin Pharmacol Ther. 2019 Aug;57(8):408-415. doi: 10.5414/CP203434. Int J Clin Pharmacol Ther. 2019. PMID: 31079600 Clinical Trial.
-
Bioequivalence Study of 100-mg Cilostazol Tablets in Healthy Thai Adult Volunteers.Curr Ther Res Clin Exp. 2019 Jul 15;91:11-16. doi: 10.1016/j.curtheres.2019.06.004. eCollection 2019. Curr Ther Res Clin Exp. 2019. PMID: 31372190 Free PMC article.
-
Pharmacokinetic comparison of sustained- and immediate-release oral formulations of cilostazol in healthy Korean subjects: a randomized, open-label, 3-part, sequential, 2-period, crossover, single-dose, food-effect, and multiple-dose study.Clin Ther. 2011 Dec;33(12):2038-53. doi: 10.1016/j.clinthera.2011.10.024. Epub 2011 Nov 29. Clin Ther. 2011. PMID: 22129569 Clinical Trial.
-
Development of a Physiologically Relevant Population Pharmacokinetic in Vitro-in Vivo Correlation Approach for Designing Extended-Release Oral Dosage Formulation.Mol Pharm. 2017 Jan 3;14(1):53-65. doi: 10.1021/acs.molpharmaceut.6b00677. Epub 2016 Dec 12. Mol Pharm. 2017. PMID: 27809538
-
Pharmacokinetics of Ambroxol Sustained Release (Mucosolvan® Retard) Compared with Other Formulations in Healthy Volunteers.Pulm Ther. 2020 Jun;6(1):119-130. doi: 10.1007/s41030-020-00116-7. Epub 2020 May 5. Pulm Ther. 2020. PMID: 32372294 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

