Development and Characterization of a Microemulsion Containing a Cannabidiol Oil and a Hydrophilic Extract from Sambucus ebulus for Topical Administration
- PMID: 38931831
- PMCID: PMC11206346
- DOI: 10.3390/pharmaceutics16060705
Development and Characterization of a Microemulsion Containing a Cannabidiol Oil and a Hydrophilic Extract from Sambucus ebulus for Topical Administration
Abstract
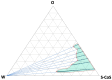
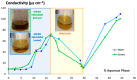
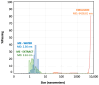
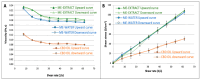
Cannabidiol (CBD) is a safe and non-psychotropic phytocannabinoid with a wide range of potential therapeutic anti-inflamatory and antioxidant activities. Due to its lipophilicity, it is normally available dissolved in oily phases. The main aim of this work was to develop and characterize a new formulation of a microemulsion with potential anti-inflammatory and antioxidant activity for the topical treatment of inflammatory skin disorders. The microemulsion system was composed of a 20% CBD oil, which served as the hydrophobic phase; Labrasol/Plurol Oleique (1:1), which served as surfactant and cosurfactant (S/CoS), respectively; and an aqueous vegetal extract obtained from Sambucus ebulus L. (S. ebulus) ripe fruits, which has potential anti-oxidant and anti-inflammatory activity and which served as the aqueous phase. A pseudo-ternary phase diagram was generated, leading to the selection of an optimal proportion of 62% (S/CoS), 27% CBD oil and 11% water and, after its reproducibility was tested, the aqueous phases were replaced by the vegetal hydrophilic extract. The defined systems were characterized in terms of conductivity, droplet size (by laser scattering), compatibility of components (by differential scanning calorimetry) and rheological properties (using a rotational rheometer). The designed microemulsion showed good stability and slight pseudo-plastic behavior. The release properties of CBD from the oil phase and caffeic acid from the aqueous phase of the microemulsion were studied via in vitro diffusion experiments using flow-through diffusion cells and were compared to those of a CBD oil and a microemulsion containing only CBD as an active substance. It was found that the inclusion of the original oil in microemulsions did not result in a significant modification of the release of CBD, suggesting the possibility of including hydrophilic active compounds in the formulation and establishing an interesting strategy for the development of future formulations.
Keywords: cannabidiol; diffusion studies; microemulsion; phytocannabinoids; rheological properties; vegetal extract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Preparation and evaluation of aceclofenac topical microemulsion.Iran J Pharm Res. 2010 Winter;9(1):5-11. Iran J Pharm Res. 2010. PMID: 24363700 Free PMC article.
-
The influence of cosurfactants and oils on the formation of pharmaceutical microemulsions based on PEG-8 caprylic/capric glycerides.Int J Pharm. 2008 Mar 20;352(1-2):231-9. doi: 10.1016/j.ijpharm.2007.10.041. Epub 2007 Nov 4. Int J Pharm. 2008. PMID: 18068919
-
Investigation of surfactant/cosurfactant synergism impact on ibuprofen solubilization capacity and drug release characteristics of nonionic microemulsions.Int J Pharm. 2012 Aug 20;433(1-2):25-33. doi: 10.1016/j.ijpharm.2012.04.070. Epub 2012 May 3. Int J Pharm. 2012. PMID: 22579578
-
Preparation and characterization of dexamethasone microemulsion based on pseudoternary phase diagram.Jundishapur J Nat Pharm Prod. 2013 Aug;8(3):105-12. doi: 10.17795/jjnpp-9373. Epub 2013 Jul 20. Jundishapur J Nat Pharm Prod. 2013. PMID: 24624198 Free PMC article.
-
Antipsoriatic microemulsion gel formulations for topical drug delivery of babchi oil (Psoralea corylifolia).Methods Find Exp Clin Pharmacol. 2008 May;30(4):277-85. doi: 10.1358/mf.2008.30.4.1185802. Methods Find Exp Clin Pharmacol. 2008. PMID: 18773122
References
-
- Evers A.W.M., Lu Y., Duller P., Van der Valk P.G.M., Kraaimaat F.W., Van de Kerkhof P.C.M. Common burden of chronic skin diseases? Contributors to psychological distress in adults with psoriasis and atopic dermatitis. Br. J. Dermatol. 2005;152:1275–1281. doi: 10.1111/j.1365-2133.2005.06565.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

