Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage
- PMID: 38931856
- PMCID: PMC11207028
- DOI: 10.3390/pharmaceutics16060732
Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage
Abstract
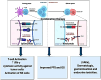
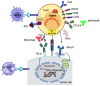
Combining immune checkpoint inhibitors, specifically nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4), holds substantial promise in revolutionizing cancer treatment. This review explores the transformative impact of these combinations, emphasizing their potential for enhancing therapeutic outcomes across various cancers. Immune checkpoint proteins, such as PD1 and CTLA4, play a pivotal role in modulating immune responses. Blocking these checkpoints unleashes anticancer activity, and the synergy observed when combining multiple checkpoint inhibitors underscores their potential for enhanced efficacy. Nivolumab and ipilimumab harness the host's immune system to target cancer cells, presenting a powerful approach to prevent tumor development. Despite their efficacy, immune checkpoint inhibitors are accompanied by a distinct set of adverse effects, particularly immune-related adverse effects affecting various organs. Understanding these challenges is crucial for optimizing treatment strategies and ensuring patient well-being. Ongoing clinical trials are actively exploring the combination of checkpoint inhibitory therapies, aiming to decipher their synergistic effects and efficacy against diverse cancer types. This review discusses the mechanisms, adverse effects, and various clinical trials involving nivolumab and ipilimumab across different cancers, emphasizing their transformative impact on cancer treatment.
Keywords: CTLA-4; PDL-1; advanced melanoma; immune subtypes; ipilimumab; molecular pathways; nivolumab; potentially malignant tumors; translational genomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials.Int J Mol Sci. 2020 Jun 22;21(12):4427. doi: 10.3390/ijms21124427. Int J Mol Sci. 2020. PMID: 32580338 Free PMC article. Review.
-
Immune checkpoint inhibitors-associated risk of immune-related hypothyroidism in older patients with advanced melanoma: a real-world analysis of US SEER-Medicare data.Expert Opin Drug Saf. 2021 Apr;20(4):489-497. doi: 10.1080/14740338.2021.1877272. Epub 2021 Jan 25. Expert Opin Drug Saf. 2021. PMID: 33445985
-
Systematic review and meta-analysis efficacy and safety of immune checkpoint inhibitors in advanced melanoma patients with anti-PD-1 progression: a systematic review and meta-analysis.Clin Transl Oncol. 2021 Sep;23(9):1885-1904. doi: 10.1007/s12094-021-02598-6. Epub 2021 Apr 20. Clin Transl Oncol. 2021. PMID: 33877531
-
Gastrointestinal adverse events with combination of checkpoint inhibitors in advanced melanoma: a systematic review.Melanoma Manag. 2018 Jan 18;5(1):MMT01. doi: 10.2217/mmt-2017-0027. eCollection 2018 Jun. Melanoma Manag. 2018. PMID: 30190927 Free PMC article. Review.
Cited by
-
Deciphering molecular landscape of breast cancer progression and insights from functional genomics and therapeutic explorations followed by in vitro validation.Sci Rep. 2024 Nov 20;14(1):28794. doi: 10.1038/s41598-024-80455-6. Sci Rep. 2024. PMID: 39567714 Free PMC article.
-
Immune evasion in cancer: mechanisms and cutting-edge therapeutic approaches.Signal Transduct Target Ther. 2025 Jul 31;10(1):227. doi: 10.1038/s41392-025-02280-1. Signal Transduct Target Ther. 2025. PMID: 40739089 Free PMC article. Review.
-
Role of Immunotherapy in Ovarian Cancer: Advances, Challenges, and Future Perspectives.Cancer Treat Res. 2025;129:187-220. doi: 10.1007/978-3-031-97242-3_10. Cancer Treat Res. 2025. PMID: 40847235 Review.
References
-
- Wang D.Y., Salem J.E., Cohen J.V., Chandra S., Menzer C., Ye F., Zhao S., Das S., Beckermann K.E., Ha L., et al. Fatal Toxic Effects Associated with Immune Checkpoint In-hibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. - DOI - PMC - PubMed
-
- Weber J.S., D’Angelo S.P., Minor D., Hodi F.S., Gutzmer R., Neyns B., Hoeller C., Khushalani N.I., Miller W.H., Jr., Lao C.D., et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/s1470-2045(15)70076-8. - DOI - PubMed
-
- Albiges L., Tannir N.M., Burotto M., McDermott D., Plimack E.R., Barthélémy P., Porta C., Powles T., Donskov F., George S., et al. First-line Nivolumab plus Ipilimumab Versus Sunitinib in Patients Without Nephrectomy and With an Eval-uable Primary Renal Tumor in the CheckMate 214 Trial. Eur. Urol. 2022;81:266–271. doi: 10.1016/j.eururo.2021.10.001. - DOI - PMC - PubMed
-
- Albiges L., Tannir N.M., Burotto M., McDermott D., Plimack E.R., Barthélémy P., Porta C., Powles T., Donskov F., George S., et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5:e001079. doi: 10.1136/esmoopen-2020-001079. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

