Development of neffy, an Epinephrine Nasal Spray, for Severe Allergic Reactions
- PMID: 38931932
- PMCID: PMC11207568
- DOI: 10.3390/pharmaceutics16060811
Development of neffy, an Epinephrine Nasal Spray, for Severe Allergic Reactions
Abstract
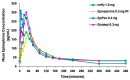
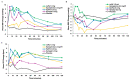
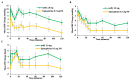
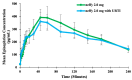
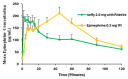
Epinephrine autoinjectors (EAIs) are used for the treatment of severe allergic reactions in a community setting; however, their utility is limited by low prescription fulfillment rates, failure to carry, and failure to use due to fear of needles. Given that delayed administration of epinephrine is associated with increased morbidity/mortality, there has been a growing interest in developing needle-free, easy-to-use delivery devices. neffy (epinephrine nasal spray) consists of three Food and Drug Administration (FDA)-approved components: epinephrine, Intravail A3 (absorption enhancer), and a Unit Dose Spray (UDS). neffy's development pathway was established in conjunction with the FDA and the European Medicines Agency and included multiple clinical trials to evaluate pharmacokinetic and pharmacodynamic responses under a variety of conditions, such as self-administration and allergic and infectious rhinitis, as well as an animal anaphylaxis model of severe hypotension, where neffy demonstrated a pharmacokinetic profile that is within the range of approved injection products and a pharmacodynamic response that is as good or better than injections. The increased pulse rate (PR) and blood pressure (BP) observed even one minute following the administration of neffy confirm the activation of α and β adrenergic receptors, which are the key components of epinephrine's mechanism of action. The results suggest that neffy will provide a safe and effective needle-free option for the treatment of severe allergic reactions, including anaphylaxis.
Keywords: anaphylaxis; epinephrine; food allergy; intranasal epinephrine; nasal spray; out of hospital use; pharmacodynamics; pharmacokinetics; severe allergy.
Conflict of interest statement
A. K. Ellis advisory boards for ALK-Abello, AstraZeneca, Aralez, Bausch Health, Leo Pharma, Merck, Novartis, and Pfizer; speakers’ bureaus for ALK-Abello, AstraZeneca, Miravo, Medexus, and Mylan; research support (paid to institution) from ALK-Abello, Aralez, AstraZeneca, Bayer LLC, Medexus, Novartis, and Regeneron; independent consultant to Bayer LLC and Regeneron; and royalties from UpToDate. She reports serving as a consultant and/or advisor for ARS Pharmaceuticals. TB Casale reports on financial support from Thermo Fisher Scientific and serves as a consultant and/or advisor for ARS Pharmaceuticals. M. Kaliner reports serving as a consultant and/or adviser for ARS Pharmaceuticals. J. Oppenheimer reports serving as a consultant/advisor for Aimmune, Amgen, ARS Pharmaceuticals, Aquestive Therapeutics, and GlaxoSmithKline; on the adjudication/DSMB for AbbVie, AstraZeneca, GlaxoSmithKline, and Sanofi; reviewer for UpToDate; executive editor for Annals of Allergy, Asthma, and Immunology. J. Spergel reports DBV Technologies, Regeneron, Sanofi, and Aimmune. He reports serving as a consultant and/or advisor for ARS Pharmaceuticals. D. Fleischer reports grants from ARS Pharmaceuticals and grants and consultant fees from DBV Technologies and serving as consultant/advisor for Aquestive Therapeutics and for Bryn Pharma. D. Bernstein has received grant support from Aimmune, ALK-Abelló, Amgen, AstraZeneca, Avillion, Biocryst, Genentech, GlaxoSmithKline, Leo, Novartis, Novum, Regeneron, Shire, and TEVA and served as an advisor for ALK America, Aquestive Therapeutics, Gerson-Lehman, GlaxoSmithKline, and Guidepoint Global. He reports serving as a consultant and/or advisor for ARS Pharmaceuticals. C. Camargo reports serving as consultant/advisor for ARS Pharmaceuticals, Bryn Pharma, and Aquestive Therapeutics. R. Lowenthal and S. Tanimoto are employees of ARS Pharmaceuticals.
Figures






References
-
- Misra S.N., Sperling M.R., Rao V.R., Peters J.M., Penovich P., Wheless J., Hogan R.E., Davis C.S., Carrazana E., Rabinowicz A.L. Analyses of patients who self-administered diazepam nasal spray for acute treatment of seizure clusters. Epilepsy Behav. Rep. 2024;25:100644. doi: 10.1016/j.ebr.2024.100644. - DOI - PMC - PubMed
-
- Cornett E.M., Nemomsa M.A., Turbeville B., Busby M.A., Kaye J.S., Kaye A.J., Choi J., Ramírez G.F., Varrassi G., Kaye A.M., et al. Midazolam nasal spray to treat intermittent, stereotypic episodes of frequent seizure activity: Pharmacology and clinical role, a comprehensive review. Health Psychol. Res. 2022;10:38536. doi: 10.52965/001c.38536. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

