In Vitro and In Vivo Antipsoriatic Efficacy of Protected and Unprotected Sugar-Zinc Phthalocyanine Conjugates
- PMID: 38931958
- PMCID: PMC11207564
- DOI: 10.3390/pharmaceutics16060838
In Vitro and In Vivo Antipsoriatic Efficacy of Protected and Unprotected Sugar-Zinc Phthalocyanine Conjugates
Abstract
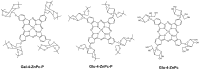
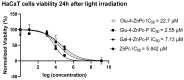
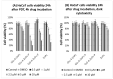
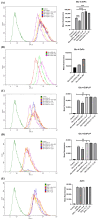
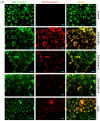
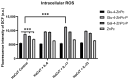
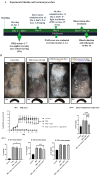
Psoriasis, a chronic immune-mediated skin disorder affecting over 125 million people globally, is characterized by abnormal keratinocyte proliferation and immune cell infiltration. Photodynamic therapy (PDT) remains underutilized in the treatment of psoriasis despite its potential as a promising and effective therapeutic approach. This study aimed to explore the efficacy of zinc phthalocyanine (ZnPc) and its sugar conjugates as potential antipsoriatic agents. We successfully synthesized protected and unprotected sugar-conjugated zinc phthalocyanines and evaluated their potential against cytokine-stimulated HaCaT keratinocytes, as well as an established IMQ psoriasis-like in vivo model. Tetrasubstituted protected glucose-ZnPc (Glu-4-ZnPc-P) demonstrated superior phototoxicity (IC50 = 2.55 µM) compared to unprotected glucose conjugate (IC50 = 22.7 µM), protected galactose-ZnPc (IC50 = 7.13 µM), and free ZnPc in cytokine-stimulated HaCaT cells (IC50 = 5.84 µM). Cellular uptake analysis revealed that IL-17A, a cytokine that plays a central role in the pathogenesis of psoriasis, enhanced unprotected Glu-4-ZnPc uptake by 56.3%, while GLUT1 inhibitor BAY-876 reduced its accumulation by 23.8%. Intracellular ROS generation following Glu-4-ZnPc-P-PDT was significantly increased after stimulation with IL-17A, correlating with in vitro photocytotoxicity. In vivo PDT using Glu-4-ZnPc-P exhibited significant improvement in Psoriasis Area and Severity Index (PASI), inhibiting splenomegaly and restoring normal skin morphology. This study highlights sugar-conjugated zinc phthalocyanines as potential candidates for targeted PDT in psoriasis, providing a basis for further clinical investigations.
Keywords: cytokines; drug uptake; psoriasis; sugar conjugates; zinc phthalocyanine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Anti-Psoriasis Effects and Mechanisms of Α-(8-Quinolinoxy) Zinc Phthalocyanine-Mediated Photodynamic Therapy.Cell Physiol Biochem. 2017;44(1):200-214. doi: 10.1159/000484647. Epub 2017 Nov 9. Cell Physiol Biochem. 2017. PMID: 29131032
-
Attritional evaluation of lipophilic and hydrophilic metallated phthalocyanines for oncological photodynamic therapy.J Photochem Photobiol B. 2021 Mar;216:112146. doi: 10.1016/j.jphotobiol.2021.112146. Epub 2021 Jan 29. J Photochem Photobiol B. 2021. PMID: 33601256
-
The Impact of Proinflammatory Cytokines and Imiquimod on GLUT1 in HaCaT Keratinocytes - a Potential Anti-Psoriatic Therapeutic Target?Cell Physiol Biochem. 2023 Mar 22;57(2):54-62. doi: 10.33594/000000615. Cell Physiol Biochem. 2023. PMID: 36945896
-
Tetra-triethyleneoxysulfonyl substituted zinc phthalocyanine for photodynamic cancer therapy.Photodiagnosis Photodyn Ther. 2016 Mar;13:148-157. doi: 10.1016/j.pdpdt.2015.07.001. Epub 2015 Jul 7. Photodiagnosis Photodyn Ther. 2016. PMID: 26162500 Review.
-
Glycoconjugation as a Promising Treatment Strategy for Psoriasis.J Pharmacol Exp Ther. 2020 May;373(2):204-212. doi: 10.1124/jpet.119.263657. Epub 2020 Mar 10. J Pharmacol Exp Ther. 2020. PMID: 32156758 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

