In Vitro and In Vivo Drug Release from a Nano-Hydroxyapatite Reinforced Resorbable Nanofibrous Scaffold for Treating Female Pelvic Organ Prolapse
- PMID: 38932015
- PMCID: PMC11207985
- DOI: 10.3390/polym16121667
In Vitro and In Vivo Drug Release from a Nano-Hydroxyapatite Reinforced Resorbable Nanofibrous Scaffold for Treating Female Pelvic Organ Prolapse
Abstract
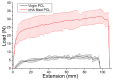
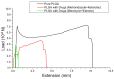
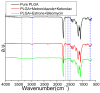
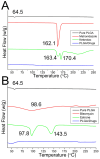
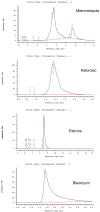
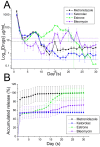
Pelvic prolapse stands as a substantial medical concern, notably impacting a significant segment of the population, predominantly women. This condition, characterized by the descent of pelvic organs, such as the uterus, bladder, or rectum, from their normal positions, can lead to a range of distressing symptoms, including pelvic pressure, urinary incontinence, and discomfort during intercourse. Clinical challenges abound in the treatment landscape of pelvic prolapse, stemming from its multifactorial etiology and the diverse array of symptoms experienced by affected individuals. Current treatment options, while offering relief to some extent, often fall short in addressing the full spectrum of symptoms and may pose risks of complications or recurrence. Consequently, there exists a palpable need for innovative solutions that can provide more effective, durable, and patient-tailored interventions for pelvic prolapse. We manufactured an integrated polycaprolactone (PCL) mesh, reinforced with nano-hydroxyapatite (nHA), along with drug-eluting poly(lactic-co-glycolic acid) (PLGA) nanofibers for a prolapse scaffold. This aims to offer a promising avenue for enhanced treatment outcomes and improved quality of life for individuals grappling with pelvic prolapse. Solution extrusion additive manufacturing and electrospinning methods were utilized to prepare the nHA filled PCL mesh and drug-incorporated PLGA nanofibers, respectively. The pharmaceuticals employed included metronidazole, ketorolac, bleomycin, and estrone. Properties of fabricated resorbable scaffolds were assessed. The in vitro release characteristics of various pharmaceuticals from the meshes/nanofibers were evaluated. Furthermore, the in vivo drug elution pattern was also estimated on a rat model. The empirical data show that nHA reinforced PCL mesh exhibited superior mechanical strength to virgin PCL mesh. Electrospun resorbable nanofibers possessed diameters ranging from 85 to 540 nm, and released effective metronidazole, ketorolac, bleomycin, and estradiol, respectively, for 9, 30, 3, and over 30 days in vitro. Further, the mesh/nanofiber scaffolds also liberated high drug levels at the target site for more than 28 days in vivo, while the drug concentrations in blood remained low. This discovery suggests that resorbable scaffold can serve as a viable option for treating female pelvic organ prolapse.
Keywords: drug-embedded nanofibers; resorbable meshes; sustained release.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Fabrication of Drug-Eluting Polycaprolactone/poly(lactic-co-glycolic Acid) Prolapse Mats Using Solution-Extrusion 3D Printing and Coaxial Electrospinning Techniques.Polymers (Basel). 2021 Jul 13;13(14):2295. doi: 10.3390/polym13142295. Polymers (Basel). 2021. PMID: 34301052 Free PMC article.
-
Hybrid Resorbable 3D-Printed Mesh/Electrospun Nanofibrous Drug/Biomolecule-Eluting Mats for Alveolar Ridge Preservation.Polymers (Basel). 2023 Aug 18;15(16):3445. doi: 10.3390/polym15163445. Polymers (Basel). 2023. PMID: 37631502 Free PMC article.
-
A novel tropoelastin-based resorbable surgical mesh for pelvic organ prolapse repair.Mater Today Bio. 2020 Oct 13;8:100081. doi: 10.1016/j.mtbio.2020.100081. eCollection 2020 Sep. Mater Today Bio. 2020. PMID: 33210083 Free PMC article.
-
Diagnosis and management of complications following pelvic organ prolapse surgery using a synthetic mesh: French national guidelines for clinical practice.Eur J Obstet Gynecol Reprod Biol. 2024 Mar;294:170-179. doi: 10.1016/j.ejogrb.2024.01.015. Epub 2024 Jan 17. Eur J Obstet Gynecol Reprod Biol. 2024. PMID: 38280271 Review.
-
Consensus Statement of the European Urology Association and the European Urogynaecological Association on the Use of Implanted Materials for Treating Pelvic Organ Prolapse and Stress Urinary Incontinence.Eur Urol. 2017 Sep;72(3):424-431. doi: 10.1016/j.eururo.2017.03.048. Epub 2017 Apr 14. Eur Urol. 2017. PMID: 28413126 Review.
Cited by
-
Hyaluronic acid-loaded drug-eluting nanofibrous pad for the treatment of degenerative arthritis.RSC Adv. 2025 May 12;15(20):15505-15515. doi: 10.1039/d5ra01494h. eCollection 2025 May 12. RSC Adv. 2025. PMID: 40365220 Free PMC article.
-
Synergistic Effects of Radical Distributions of Soluble and Insoluble Polymers within Electrospun Nanofibers for an Extending Release of Ferulic Acid.Polymers (Basel). 2024 Sep 15;16(18):2614. doi: 10.3390/polym16182614. Polymers (Basel). 2024. PMID: 39339078 Free PMC article.
-
Evaluation of Electrospun Poly-4-Hydroxybutyrate as Biofunctional and Degradable Scaffold for Pelvic Organ Prolapse in a Vaginal Sheep Model.Macromol Biosci. 2025 Apr;25(4):e2400412. doi: 10.1002/mabi.202400412. Epub 2025 Feb 26. Macromol Biosci. 2025. PMID: 40008865 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

