Development of a Fully Protective Pandemic Avian Influenza Subunit Vaccine in Insect Pupae
- PMID: 38932122
- PMCID: PMC11209067
- DOI: 10.3390/v16060829
Development of a Fully Protective Pandemic Avian Influenza Subunit Vaccine in Insect Pupae
Abstract
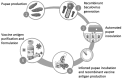
In this study, we pioneered an alternative technology for manufacturing subunit influenza hemagglutinin (HA)-based vaccines. This innovative method involves harnessing the pupae of the Lepidoptera Trichoplusia ni (T. ni) as natural biofactories in combination with baculovirus vectors (using CrisBio® technology). We engineered recombinant baculoviruses encoding two versions of the HA protein (trimeric or monomeric) derived from a pandemic avian H7N1 virus A strain (A/chicken/Italy/5093/99). These were then used to infect T. ni pupae, resulting in the production of the desired recombinant antigens. The obtained HA proteins were purified using affinity chromatography, consistently yielding approximately 75 mg/L of insect extract. The vaccine antigen effectively immunized poultry, which were subsequently challenged with a virulent H7N1 avian influenza virus. Following infection, all vaccinated animals survived without displaying any clinical symptoms, while none of the mock-vaccinated control animals survived. The CrisBio®-derived antigens induced high titers of HA-specific antibodies in the vaccinated poultry, demonstrating hemagglutination inhibition activity against avian H7N1 and human H7N9 viruses. These results suggest that the CrisBio® technology platform has the potential to address major industry challenges associated with producing recombinant influenza subunit vaccines, such as enhancing production yields, scalability, and the speed of development, facilitating the global deployment of highly effective influenza vaccines.
Keywords: Trichoplusia ni; baculovirus; insect pupae; pandemic influenza; subunit vaccine.
Conflict of interest statement
Authors Susana Martínez, Maria del Carmen Nuñez, Edel Reytor, Miguel Cid, Ana Falcón, and José M. Escribano were employed by the company Algenex. The remaining authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
References
-
- Kumar N., Pandey N., Halder A. Preventive, diagnostic and therapeutic application of baculovirus expression vector system. In: Kumar D., Gong C., editors. Trends in Insect Molecular Biology and Biotechnology. Springer; Cham, Switzerland: 2018. pp. 163–191.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

