Acute and Long COVID Intestinal Changes in an Experimental Model of Coronavirus in Mice
- PMID: 38932125
- PMCID: PMC11209276
- DOI: 10.3390/v16060832
Acute and Long COVID Intestinal Changes in an Experimental Model of Coronavirus in Mice
Abstract
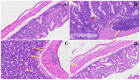
The COVID-19 pandemic, which emerged in early 2020, has had a profound and lasting impact on global health, resulting in over 7.0 million deaths and persistent challenges. In addition to acute concerns, there is growing attention being given to the long COVID health consequences for survivors of COVID-19 with documented cases of cardiovascular abnormalities, liver disturbances, lung complications, kidney issues, and noticeable cognitive deficits. Recent studies have investigated the physiological changes in various organs following prolonged exposure to murine hepatitis virus-1 (MHV-1), a coronavirus, in mouse models. One significant finding relates to the effects on the gastrointestinal tract, an area previously understudied regarding the long-lasting effects of COVID-19. This research sheds light on important observations in the intestines during both the acute and the prolonged phases following MHV-1 infection, which parallel specific changes seen in humans after exposure to SARS-CoV-2. Our study investigates the histopathological alterations in the small intestine following MHV-1 infection in murine models, revealing significant changes reminiscent of inflammatory bowel disease (IBD), celiac disease. Notable findings include mucosal inflammation, lymphoid hyperplasia, goblet cell hyperplasia, and immune cell infiltration, mirroring pathological features observed in IBD. Additionally, MHV-1 infection induces villous atrophy, altered epithelial integrity, and inflammatory responses akin to celiac disease and IBD. SPIKENET (SPK) treatment effectively mitigates intestinal damage caused by MHV-1 infection, restoring tissue architecture and ameliorating inflammatory responses. Furthermore, investigation into long COVID reveals intricate inflammatory profiles, highlighting the potential of SPK to modulate intestinal responses and restore tissue homeostasis. Understanding these histopathological alterations provides valuable insights into the pathogenesis of COVID-induced gastrointestinal complications and informs the development of targeted therapeutic strategies.
Keywords: fibrosis; goblet cell; infection; intestine; long COVID; murine hepatitis virus-1; pili.
Conflict of interest statement
Paidas is a Scientific Advisory Board Member of BioIncept, LLC, with stock options. The other authors have no conflicts of interest or competing interests to declare.
Figures
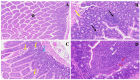

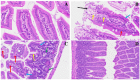
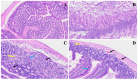


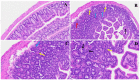
References
-
- World Health Organization . Who Coronavirus (COVID-19) Dashboard. World Health Organization; Geneva, Switzerland: [(accessed on 20 July 2023)]. Available online: https://covid19.who.int/
-
- Sullivan M.K., Lees J.S., Drake T.M., Docherty A.B., Oates G., Hardwick H.E., Russell C.D., Merson L., Dunning J., Nguyen-Van-Tam J.S., et al. Acute Kidney Injury in Patients Hospitalized With COVID-19 From the ISARIC WHO CCP-UK Study: A Prospective, Multicentre Cohort Study. Nephrol. Dial. Transplant. 2022;37:271–284. doi: 10.1093/ndt/gfab303. - DOI - PMC - PubMed
-
- Mortaz E., Tabarsi P., Jamaati H., Dalil Roofchayee N., Dezfuli N.K., Hashemian S.M., Moniri A., Marjani M., Malekmohammad M., Mansouri D., et al. Increased Serum Levels of Soluble TNF-α Receptor Is Associated with ICU Mortality in COVID-19 Patients. Front. Immunol. 2021;12:592727. doi: 10.3389/fimmu.2021.592727. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

