Whole-Genome Sequencing and Genetic Diversity of Human Respiratory Syncytial Virus in Patients with Influenza-like Illness in Sicily (Italy) from 2017 to 2023
- PMID: 38932144
- PMCID: PMC11209242
- DOI: 10.3390/v16060851
Whole-Genome Sequencing and Genetic Diversity of Human Respiratory Syncytial Virus in Patients with Influenza-like Illness in Sicily (Italy) from 2017 to 2023
Abstract
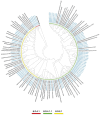
Monitoring the genetic variability of human respiratory syncytial virus (hRSV) is of paramount importance, especially for the potential implication of key antigenic mutations on the emergence of immune escape variants. Thus, to describe the genetic diversity and evolutionary dynamics of hRSV circulating in Sicily (Italy), a total of 153 hRSV whole-genome sequences collected from 770 hRSV-positive subjects between 2017 and 2023, before the introduction of expanded immunization programs into the population, were investigated. The phylogenetic analyses indicated that the genotypes GA.2.3.5 (ON1) for hRSV-A and GB.5.0.5a (BA9) for hRSV-B co-circulated in our region. Amino acid (AA) substitutions in the surface and internal proteins were evaluated, including the F protein antigenic sites, as the major targets of immunoprophylactic monoclonal antibodies and vaccines. Overall, the proportion of AA changes ranged between 1.5% and 22.6% among hRSV-A, whereas hRSV-B varied in the range 0.8-16.9%; the latter was more polymorphic than hRSV-A within the key antigenic sites. No AA substitutions were found at site III of both subgroups. Although several non-synonymous mutations were found, none of the polymorphisms known to potentially affect the efficacy of current preventive measures were documented. These findings provide new insights into the global hRSV molecular epidemiology and highlight the importance of defining a baseline genomic picture to monitor for future changes that might be induced by the selective pressures of immunological preventive measures, which will soon become widely available.
Keywords: Italy; Sicily; amino acid change; community; mAb; molecular surveillance; respiratory syncytial virus; vaccine; whole-genome sequencing.
Conflict of interest statement
All authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures




References
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., Madhi S.A., Omer S.B., Simões E.A.F., Campbell H., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
-
- Díez-Gandía E., Gómez-Álvarez C., López-Lacort M., Muñoz-Quiles C., Úbeda-Sansano I., Díez-Domingo J., Orrico-Sánchez A., Study Collaborators The impact of childhood RSV infection on children’s and parents’ quality of life: A prospective multicenter study in Spain. BMC Infect. Dis. 2021;21:924. doi: 10.1186/s12879-021-06629-z. - DOI - PMC - PubMed
-
- Tramuto F., Maida C.M., Di Naro D., Randazzo G., Vitale F., Restivo V., Costantino C., Amodio E., Casuccio A., Graziano G., et al. Respiratory Syncytial Virus: New Challenges for Molecular Epidemiology Surveillance and Vaccination Strategy in Patients with ILI/SARI. Vaccines. 2021;9:1334. doi: 10.3390/vaccines9111334. - DOI - PMC - PubMed
-
- Nguyen-Van-Tam J.S., O’Leary M., Martin E.T., Heijnen E., Callendret B., Fleischhackl R., Comeaux C., Tran T.M.P., Weber K. Burden of respiratory syncytial virus infection in older and high-risk adults: A systematic review and meta-analysis of the evidence from developed countries. Eur. Respir. Rev. 2022;31:220105. doi: 10.1183/16000617.0105-2022. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

