A 44-Nucleotide Region in the Chikungunya Virus 3' UTR Dictates Viral Fitness in Disparate Host Cells
- PMID: 38932154
- PMCID: PMC11209300
- DOI: 10.3390/v16060861
A 44-Nucleotide Region in the Chikungunya Virus 3' UTR Dictates Viral Fitness in Disparate Host Cells
Abstract
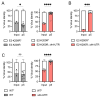
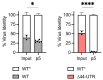
We previously reported that deletion of a 44-nucleotide element in the 3' untranslated region (UTR) of the Chikungunya virus (CHIKV) genome enhances the virulence of CHIKV infection in mice. Here, we find that while this 44-nucleotide deletion enhances CHIKV fitness in murine embryonic fibroblasts in a manner independent of the type I interferon response, the same mutation decreases viral fitness in C6/36 mosquito cells. Further, the fitness advantage conferred by the UTR deletion in mammalian cells is maintained in vivo in a mouse model of CHIKV dissemination. Finally, SHAPE-MaP analysis of the CHIKV 3' UTR revealed this 44-nucleotide element forms a distinctive two-stem-loop structure that is ablated in the mutant 3' UTR without altering additional 3' UTR RNA secondary structures.
Keywords: 3′ UTR; CHIKV; Chikungunya virus; RNA structure; SHAPE-MaP; viral fitness.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Morrison T.E., Ander S.E. Encyclopedia of Virology. Volume 2. Elsevier; Amsterdam, The Netherlands: 2021. Chikungunya Virus (Togaviridae) pp. 173–181.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

