Cationic Residues of the HIV-1 Nucleocapsid Protein Enable DNA Condensation to Maintain Viral Core Particle Stability during Reverse Transcription
- PMID: 38932164
- PMCID: PMC11209390
- DOI: 10.3390/v16060872
Cationic Residues of the HIV-1 Nucleocapsid Protein Enable DNA Condensation to Maintain Viral Core Particle Stability during Reverse Transcription
Abstract
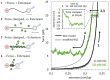
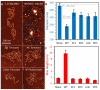
The HIV-1 nucleocapsid protein (NC) is a multifunctional viral protein necessary for HIV-1 replication. Recent studies have demonstrated that reverse transcription (RT) completes in the intact viral capsid, and the timing of RT and uncoating are correlated. How the small viral core stably contains the ~10 kbp double stranded (ds) DNA product of RT, and the role of NC in this process, are not well understood. We showed previously that NC binds and saturates dsDNA in a non-specific electrostatic binding mode that triggers uniform DNA self-attraction, condensing dsDNA into a tight globule against extending forces up to 10 pN. In this study, we use optical tweezers and atomic force microscopy to characterize the role of NC's basic residues in dsDNA condensation. Basic residue mutations of NC lead to defective interaction with the dsDNA substrate, with the constant force plateau condensation observed with wild-type (WT) NC missing or diminished. These results suggest that NC's high positive charge is essential to its dsDNA condensing activity, and electrostatic interactions involving NC's basic residues are responsible in large part for the conformation, size, and stability of the dsDNA-protein complex inside the viral core. We observe DNA re-solubilization and charge reversal in the presence of excess NC, consistent with the electrostatic nature of NC-induced DNA condensation. Previous studies of HIV-1 replication in the presence of the same cationic residue mutations in NC showed significant defects in both single- and multiple-round viral infectivity. Although NC participates in many stages of viral replication, our results are consistent with the hypothesis that cationic residue mutations inhibit genomic DNA condensation, resulting in increased premature capsid uncoating and contributing to viral replication defects.
Keywords: DNA condensation; HIV-1 nucleocapsid protein; atomic force microscopy; capsid uncoating; optical tweezers.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

