Elucidation of the Epitranscriptomic RNA Modification Landscape of Chikungunya Virus
- PMID: 38932237
- PMCID: PMC11209572
- DOI: 10.3390/v16060945
Elucidation of the Epitranscriptomic RNA Modification Landscape of Chikungunya Virus
Abstract
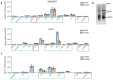
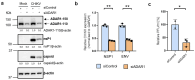
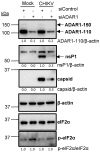
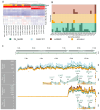
The genomes of positive-sense (+) single-stranded RNA (ssRNA) viruses are believed to be subjected to a wide range of RNA modifications. In this study, we focused on the chikungunya virus (CHIKV) as a model (+) ssRNA virus to study the landscape of viral RNA modification in infected human cells. Among the 32 distinct RNA modifications analysed by mass spectrometry, inosine was found enriched in the genomic CHIKV RNA. However, orthogonal validation by Illumina RNA-seq analyses did not identify any inosine modification along the CHIKV RNA genome. Moreover, CHIKV infection did not alter the expression of ADAR1 isoforms, the enzymes that catalyse the adenosine to inosine conversion. Together, this study highlights the importance of a multidisciplinary approach to assess the presence of RNA modifications in viral RNA genomes.
Keywords: ADAR1; RNA modifications; alphaviruses; chikungunya virus; epitranscriptome; inosine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Using SHAPE-MaP To Model RNA Secondary Structure and Identify 3'UTR Variation in Chikungunya Virus.J Virol. 2020 Nov 23;94(24):e00701-20. doi: 10.1128/JVI.00701-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999019 Free PMC article.
-
YBX1 is required for assembly of viral replication complexes of chikungunya virus and replication of multiple alphaviruses.J Virol. 2025 Feb 25;99(2):e0201524. doi: 10.1128/jvi.02015-24. Epub 2024 Dec 31. J Virol. 2025. PMID: 39745458 Free PMC article.
-
The Epitranscriptome and Innate Immunity.PLoS Genet. 2015 Dec 10;11(12):e1005687. doi: 10.1371/journal.pgen.1005687. eCollection 2015 Dec. PLoS Genet. 2015. PMID: 26658668 Free PMC article. Review.
-
A novel interaction between the 5' untranslated region of the Chikungunya virus genome and Musashi RNA binding protein is essential for efficient virus genome replication.Nucleic Acids Res. 2024 Sep 23;52(17):10654-10667. doi: 10.1093/nar/gkae619. Nucleic Acids Res. 2024. PMID: 39087525 Free PMC article.
-
Biased (A-->I) hypermutation of animal RNA virus genomes.Curr Opin Genet Dev. 1994 Dec;4(6):895-900. doi: 10.1016/0959-437x(94)90076-0. Curr Opin Genet Dev. 1994. PMID: 7888761 Review.
Cited by
-
Bioinformatics for Inosine: Tools and Approaches to Trace This Elusive RNA Modification.Genes (Basel). 2024 Jul 29;15(8):996. doi: 10.3390/genes15080996. Genes (Basel). 2024. PMID: 39202357 Free PMC article. Review.
-
Advances in Detection Methods for A-to-I RNA Editing.Wiley Interdiscip Rev RNA. 2025 Mar-Apr;16(2):e70014. doi: 10.1002/wrna.70014. Wiley Interdiscip Rev RNA. 2025. PMID: 40223708 Free PMC article. Review.
-
IFN alpha inducible protein 27 (IFI27) acts as a positive regulator of PACT-dependent PKR activation after RNA virus infections.PLoS Pathog. 2025 Jun 16;21(6):e1013246. doi: 10.1371/journal.ppat.1013246. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40522981 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

