Clinical Characteristics and Outcomes of Pediatric COVID-19 Pneumonia Treated with Favipiravir in a Tertiary Care Center
- PMID: 38932238
- PMCID: PMC11209591
- DOI: 10.3390/v16060946
Clinical Characteristics and Outcomes of Pediatric COVID-19 Pneumonia Treated with Favipiravir in a Tertiary Care Center
Abstract
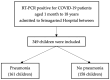
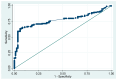
The COVID-19 pandemic, caused by SARS-CoV-2, has posed significant health challenges worldwide. While children generally experience less severe illness compared to adults, pneumonia remains a substantial risk, particularly for those under five years old. This study examines the clinical characteristics and treatment outcomes of pediatric COVID-19 pneumonia patients treated with favipiravir in Thailand, aiming to identify associated factors for pneumonia. A retrospective review was performed on pediatric patients aged 1 month to 18 years hospitalized with COVID-19 at Srinagarind Hospital, Khon Kaen University, from 13 January 2020 to 15 November 2021. Data on demographics, clinical symptoms, treatment, and outcomes were collected, and logistic regression analysis was used to identify factors associated with pneumonia. Among 349 hospitalized children, the median age was 8 years, with 51.9% being male. Symptoms included a fever (100%), a cough (74.2%), and a rash (24.9%). COVID-19 pneumonia was diagnosed in 54.7% of the children. Favipiravir was administered as the standard treatment, showing mild adverse effects, including a rash (4.3%) and nausea (2.8%). Monocytosis was significantly associated with COVID-19 pneumonia (aOR 30.85, 95% CI: 9.03-105.41, p < 0.001), with an ROC curve area of 0.77 (95% CI: 0.71-0.83). Pediatric COVID-19 patients typically exhibit mild-to-moderate symptoms, with pneumonia being common in the early pandemic phase. Monocytosis is a significant factor associated with COVID-19 pneumonia. Favipiravir demonstrated mild adverse effects. Further studies are needed to validate these findings across different settings and phases of the pandemic.
Keywords: COVID-19; children; favipiravir; monocytosis; pneumonia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Wong J.J.M., Abbas Q., Chuah S.L., Malisie R.F., Pon K.M., Katsuta T., Dang H., Lee P.C., Jayashree M., Sultana R., et al. Comparative Analysis of Pediatric COVID-19 Infection in Southeast Asia, South Asia, Japan, and China. Am. J. Trop. Med. Hyg. 2021;105:413–420. doi: 10.4269/ajtmh.21-0299. - DOI - PMC - PubMed
-
- Satdhabudha A., Chaiyakulsil C., Uppala R., Niyomkarn W., Tovichien P., Norasettekul V., Ruangnapa K., Smathakanee C., Choursamran B., Kulbun A., et al. Development and Validation of the Predictive Score for Pediatric COVID-19 Pneumonia: A Nationwide, Multicenter Study. PLoS ONE. 2022;17:e0273842. doi: 10.1371/journal.pone.0273842. - DOI - PMC - PubMed
-
- Uppala R., Sitthikarnkha P., Niamsanit S., Sutra S., Thepsuthammarat K., Techasatian L., Anantasit N., Teeratakulpisarn J. Effect of the COVID-19 Pandemic on Lower Respiratory Tract Infection Determinants in Thai Hospitalized Children: National Data Analysis 2015–2020. Trop. Med. Infect. Dis. 2022;7:151. doi: 10.3390/tropicalmed7080151. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous