Breakthrough Acute HIV Infections among Pre-Exposure Prophylaxis Users with High Adherence: A Narrative Review
- PMID: 38932243
- PMCID: PMC11209220
- DOI: 10.3390/v16060951
Breakthrough Acute HIV Infections among Pre-Exposure Prophylaxis Users with High Adherence: A Narrative Review
Abstract
Pre-exposure prophylaxis (PrEP) is a pivotal intervention among HIV prevention strategies. We aimed to narratively revise the topic of HIV acute infection in the setting of PrEP exposure with a focus on diagnostic options, clinical features, and future PrEP perspectives, with a particular focus on users with high adherence to PrEP. We searched the main databases (PubMed, Embase, and Scopus) with the keywords "PrEP" or "Pre-Exposure Prophylaxis" and "HIV" or "PLWH" and "breakthrough" or "acute infection" or "primary infection". We included all randomized clinical trials and non-experimental studies (both case reports and observational studies) ever published. In the present narrative review, we revise the diagnostic challenges related to HIV diagnosis in the setting of PrEP and the clinical characteristics and symptoms of breakthrough infections. We discuss the management of acute HIV infection during PrEP and the new challenges that arise from the use of long-acting drugs for PrEP. Our review underlines that although extremely rare, HIV seroconversions are still possible during PrEP, even in a context of high adherence. Efforts to promptly identify these events must be included in the PrEP follow-up in order to minimize the chance of overlooked HIV breakthrough infections and thus exposure to suboptimal concentrations of antiretrovirals.
Keywords: HIV; PrEP; adherence; failure; primary HIV.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to the present manuscript. D.M. has received non-financial educational support from Gilead Sciences (Foster City, CA, USA) and ViiV Healthcare (Middlesex, UK) and speaker bureau fees from ViiV Healthcare (UK), Gilead Sciences (USA), and MSD (Rahway, NJ, USA). A. Giacomelli has received consultancy fees from Mylan and Jansen and non-financial educational support and a research grant from Gilead Sciences and ViiV Healthcare. A. Gori received grants and fees for the speaker bureau, advisory boards, and CME activities from JANSSEN (Beerse, Belgium), VIIV (UK), MSD (USA), BMS (Lawrence Township, NJ, USA), ABBVIE (North Chicago, IL, USA), GILEAD (USA), NOVARTIS (Basel, Switzerland), PFIZER (New York, NY, USA), ASTELLAS (Tokyo, Japan), ASTRAZENECA (Cambridge, UK), and ANGELINI (Rome, Italy). S.A. has received support for research activities from Pfizer and Merck Sharp & Dome. The other authors have nothing to declare.
Figures

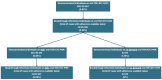
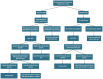

References
-
- DANGER: UNAIDS Global AIDS Update 2022. Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland: 2022. Licence: CC BY-NC-SA 3.0 IGO.
-
- Grant R.M., Lama J.R., Anderson P.L., McMahan V., Liu A.Y., Vargas L., Goicochea P., Casapía M., Guanira-Carranza J.V., Ramirez-Cardich M.E., et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N. Engl. J. Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

